Oncology and Cancer Screening
OPEN ACCESS | Volume 7 - Issue 1 - 2026
ISSN No: 2994-8746 | Journal DOI: 10.61148/2994-8746/JOCS
Faris Altom 1*, Lama S. Alahmadi 2
1Medicine, Alrayan College, Almadinah, SAU.
2Medicine and Surgery, Al-Rayan Colleges, Madina, SAU.
*Corresponding Author: Faris Altom, Medicine, Alrayan College, Almadinah, SAU.
Received: August 05, 2024
Accepted: August 08, 2024
Published: August 21, 2024
Citation: Faris Altom, Lama S. Alahmadi, Sarah A. Aloufi, Riyaa L. Alharbi, Saada S. Alharbi. (2024) “Assessment of P16 and Ki-67 Proteins Overexpression in Oral Mucosa among Saudi Smokers using Immunohistochemistry”, J Oncology and Cancer Screening, 5(1); DOI: DOI: 10.61148/2994-8746/JOCS/67
Copyright: © 2024 Faris Altom. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background: Tobacco use is well recognized as a significant contributing factor to a range of health issues, such as mouth cancer. The proteins P16 and Ki-67 have a role in regulating the cell cycle and promoting cell growth. Analyzing the levels of these proteins can give us valuable information about the overall health of cells.
Objective: This study aims is to assess the cellular alterations and immunohistochemical expression of P16 and Ki-67 in the oral mucosa of Saudi individuals who smoke.
Method: Between 2022 and 2023, a cross-sectional study scraped 500 samples from the buccal mucosa. Participants in the study were Saudi citizens of both genders. A total of 300 individuals who smoked cigarettes and 200 individuals who did not smoke tobacco were selected as the control group. The selection process involved two sample techniques: initially purposive sampling and subsequently snowball sampling. The samples underwent immunohistochemical examination to determine the presence of P16 and Ki-67 protein overexpression. We evaluated the samples by assessing the proportion of cells that exhibited positive staining and the intensity of the staining. The data were examined utilizing SPSS. We identified categorical variables by calculating frequencies and percentages using the chi-squared test. A significance level of P<0.05 was used to determine statistical significance.
Result: A total of 48% of cigarette users had abnormal results, compared to 20% of nonsmokers. These abnormalities included cytological atypia, inflammation, reverse cytological infection, and binucleated or multinucleated cells (P = 0.015). People who smoked had higher levels of P16 and Ki-67 expression than people who didn't smoke (12% and 5%, respectively) (P = 0.042). There were no important changes in P16/Ki- 67 expression between participants of different ages (P = 0.68) or between men and women (P = 0.27).
Conclusion: These results show that smoking is detrimental for cell health and stress how important it is to stop smoking to lower the chance of cytological abnormalities and diseases related to them. Smokers have higher levels of the proteins P16 and Ki-67, which shows how important these biomarkers are for knowing the risks to oral health.
Introduction
Tobacco use poses significant health risks and is a major contributor to morbidity and premature mortality globally. Five million people die every year from smoking-related illnesses, most of them in developing countries [1]. The US spends about $167 billion annually on health problems caused by smoking [2]. Similarly, Saudi Arabia spends about $160 million annually on tobacco [3].
Many studies have looked at the changes in cells in the mouth mucosa of smokers using different methods, such as cytological assessment, DNA damage detection, and histological investigations. These studies show that smoking causes many changes, such as changes in cell differentiation, greater cell proliferation, and increased epithelial thickness. In many cases, these changes occur because tobacco smoke damages DNA and causes changes in genes that either prevent or cause tumors [4, 5]. P16, also known as cyclin-dependent kinase inhibitor 2A (CDKN2A), is a tumor suppressor protein. It regulates the cell cycle and prevents cells from multiplying [6]. Several studies that looked into oral cancer [7] found that P16 was overexpressed. Conversely, Ki-67, a protein associated with cell growth and proliferation, signals these processes [8]. Numerous diseases, including oral cancer, have been associated with high levels of Ki-67 [9].
A lot of people use immunohistochemistry (IHC) to find and measure protein expression in organs. Certain antibodies bind to the target protein, while a chromogen stains the antibody-bound protein to reveal it under a microscope [10].
Researchers have extensively studied the correlation between smoking and elevated levels of P16 and Ki-67 proteins in various body parts. For example, Sunberg et al. found that people who smoke have significantly higher levels of P16 mRNA in oral squamous cell carcinoma compared to people who don't smoke [7].
Similar to this, other research has found a link between smoking and higher levels of Ki-67 in oral tumors [11, 12].
This study aims to investigate the cellular alterations and immunohistochemical expression of P16 and Ki- 67 in the oral mucosa among smokers in Saudi Arabia, providing insights into the potential biomarkers for early detection of smoking-related oral pathologies.
Materials And Methods
Study Design and Participants From:
March to December 2023, 500 randomly selected healthy volunteers participated in this cross-sectional study, with 300 cigarette smokers and 200 nonsmokers serving as the control group. With a 95% confidence level and a 5% margin of error, we used the Epi Info Software Package Version 7.2 (Centers for Disease Control and Prevention, Atlanta, Georgia) to figure out the sample number. We ensured adherence to all safety protocols and obtained two buccal smears from each subject [13]. People ages 18 to 85 who smoked or didn't smoke were both in fair health. Everyone who participated was a Saudi national of either gender and at least 18 years old. Participants could not be Saudi citizens, under 18 years old, or without informed permission.
Sample Collection:
A buccal smear is a medical procedure that involves collecting cells from inside the buccal mucosa for examination. Using a wooden tongue depressor, we obtained exfoliative cells from the oral mucosa, specifically from the tongue dorsum and both cheeks. Subsequently, we proceeded to evenly distribute the cells onto two pristine glass slides and promptly preserved them in 95% ethyl alcohol while they were still damp. We dispatched the buccal smears to the histopathology lab at Rayyan College of Medicine in Saudi Arabia for staining and diagnosis.
Papanicolaou’s Staining:
We hydrated the smears in a descending series of ethanol concentrations (diluted with distilled water) from 95% to 70% for two minutes each after fixation in ethanol. We treated the smears with Harris hematoxylin for five minutes to stain the nuclei, rinsed them in distilled water, differentiated them in 0.5% aqueous hydrochloric acid for ten seconds, and then rinsed them again in distilled water. Following a four-second blue dyeing process in alkaline water, we dehydrated the smears twice, for two minutes each, using an ascending series of ethanol concentrations from 70% to 95%. We then stained the smears with Papanicolaou Orange G6 solution for two minutes, rinsed them with 95% ethanol, incubated them with Papanicolaou EA50 staining solution for three minutes, and checked for cytoplasmic staining. Following dehydration in 95% pure ethanol, we cleared the smears in xylene and mounted them using dibutylphthalate polystyrene xylene (DPX) [13].
Immunocytochemistry:
We subjected the smears to three rounds of rinsing, each lasting three minutes, using phosphate-buffered saline (PBS). To decrease the activity of endogenous peroxidase, we applied a solution of 0.3% hydrogen peroxide in methanol to each slide for a duration of 15 minutes. We then rinsed the slides three times with PBS. Next, we exposed the slides to primary mouse monoclonal Ki-67 and P16 antibodies (Gene Tech Company Limited, Shanghai, China) at a dilution of 1/100 for 30 minutes at a temperature of 37 °C. We treated the slides with a secondary antibody, specifically Chem Mate TM EnVision+/HRP (Gene Tech Company Limited), at room temperature for 30 minutes. We then performed three PBS washes, followed by two additional PBS washes.
As the final chromogen, a 1/100 dilution of diaminobenzidine (DAB) from Gene Tech Company Limited was used to measure immunoreactivity. The chromogen was applied for 10 minutes, followed by a three-minute wash in distilled water. Ultimately, we applied a hematoxylin counterstain to the sections for a duration of three minutes. Subsequently, we rinsed the sections in running tap water for a period of five minutes, followed by dehydration in a sequence of alcoholic solutions. Afterwards, we cleaned the sections with xylene and finally mounted them using DPX. The presence of Ki-67 and P16 expression in epithelial cells and nuclei was detected through clear and specific brown cytoplasmic staining [14].
Cytological Evaluation:
We examined Pap-stained smears for cytopathological abnormalities. We looked for indications of keratinization, atypia, inflammation, and infection. Features such as uneven growth and bi- or multi- nucleation identified cytological changes [14].
Quantitative Analysis:
We conducted statistical analysis using IBM SPSS Statistics for Windows, Version 22 (published in 2013; IBM Corp., Armonk, New York), with a significance level set at 0.05. We represented categorical data as frequencies or proportions and examined the study topics and data types with chi-square testing.
Ethical Consent:
Before collecting specimens, each participant was required to complete a written ethical consent form. The Al Rayyan Medical Colleges (AMC) Ethical Committee designed and approved the informed ethical consent form (Grant No. HA-03-M-122-045).
Results
Cytological Findings
Out of the 300 cigarette smokers, 144 (48%) showed cytological abnormalities, including inflammatory cells, infection, atypia, and binucleated/multinucleated cells. In contrast, among the 200 nonsmokers, 40 (20%) exhibited similar abnormalities (P=0.015) as shown in table 1 and Figure 1.
|
Group |
Normal Cells (%) |
Abnormal Cells (%) |
|
Smokers (n=300) |
156 (52%) |
144 (48%) |
|
Nonsmokers (n=200) 160 (80%) 40 (20%) |
||
TABLE 1: Study group and cytological findings
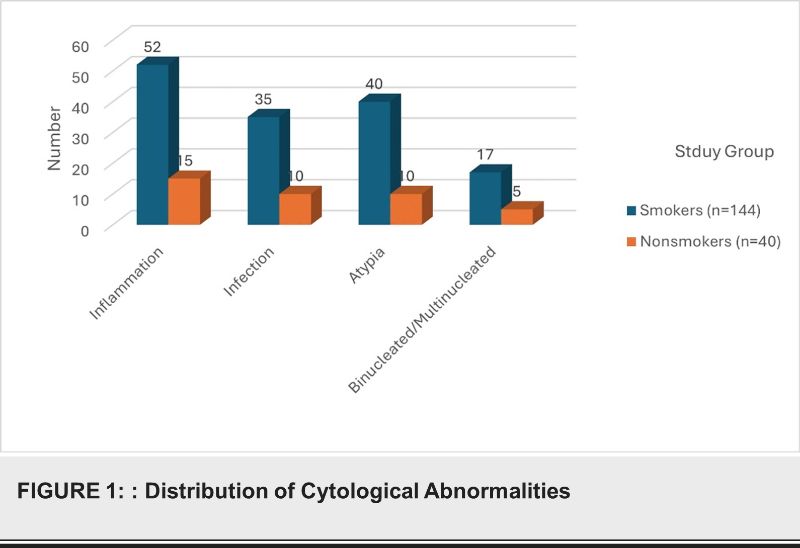
The age distribution of the participants ranged from 18 to 85 years. The study found no significant differences in P16 and Ki-67 expression across different age groups (P=0.68)as shown in table 2.
|
Age Group (years) |
Smokers (n=300) |
Nonsmokers (n=200) |
P16 Expression (%) |
Ki-67 Expression (%) |
|
18-30 |
90 |
60 |
10 (11.1%) |
5 (5.6%) |
|
31-45 |
80 |
50 |
8 (10%) |
3 (3.8%) |
|
46-60 |
70 |
40 |
9 (12.9%) |
4 (5.7%) |
|
61-75 |
40 |
30 |
5 (12.5%) |
2 (5%) |
|
76-85 |
20 |
20 |
4 (20%) |
1 (5%) |
TABLE 2: Age Distribution and Protein Expression
|
Gender |
Smokers (n=300) |
Nonsmokers (n=200) |
P16 Expression (%) |
Ki-67 Expression (%) |
|
Male (n=361) |
217 |
144 |
25 (11.5%) |
12 (5.5%) |
|
Female (n=139) |
83 |
56 |
11 (7.9%) |
7 (5%) |
Table 3. The gender distribution included 361 males and 139 females. There were no significant differences in P16 and Ki-67 expression between male and female participants (P=0.27).
The immunohistochemical analysis revealed that smokers had significantly higher expressions of P16 and Ki-67 proteins compared to nonsmokers. P16 overexpression was observed in 36 (12%) smokers and 2 (1%) nonsmokers (P=0.042). Ki-67 overexpression was detected in 15 (5%) smokers and 4 (2%) nonsmokers (P=0.042) as shown in table 4.
|
Parameter |
Smokers (n=300) |
Nonsmokers (n=200) |
P-value |
|
Abnormal Cells |
144 (48%) |
40 (20%) |
0.015 |
|
P16 Overexpression |
36 (12%) |
2 (1%) |
0.042 |
|
Ki-67 Overexpression |
15 (5%) |
4 (2%) |
0.042 |
TABLE 4: Immunohistochemical expression and study group
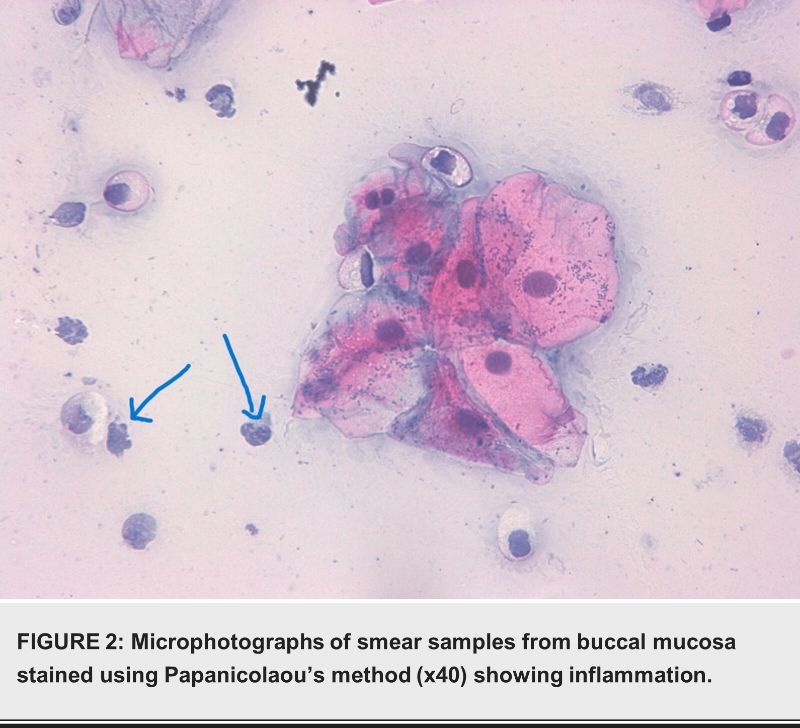
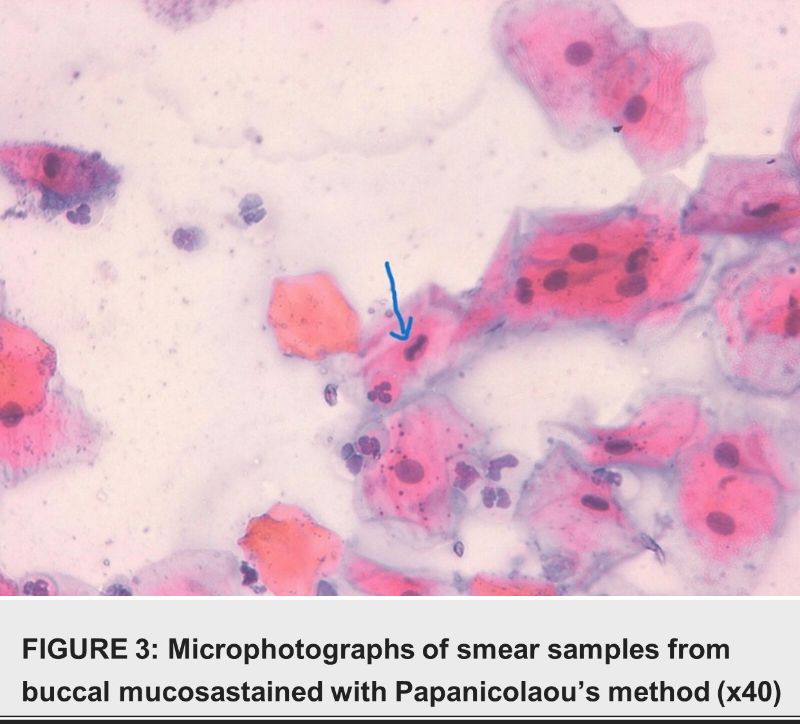
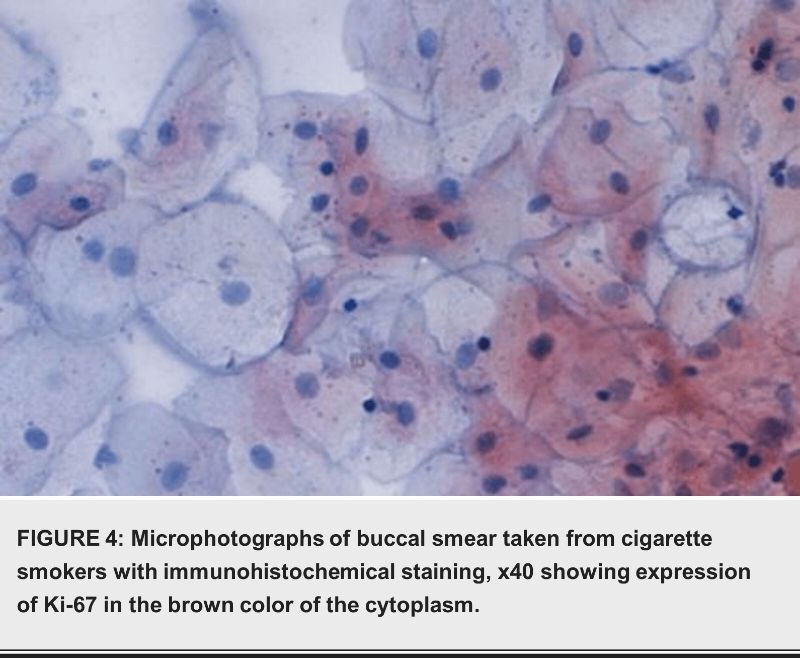
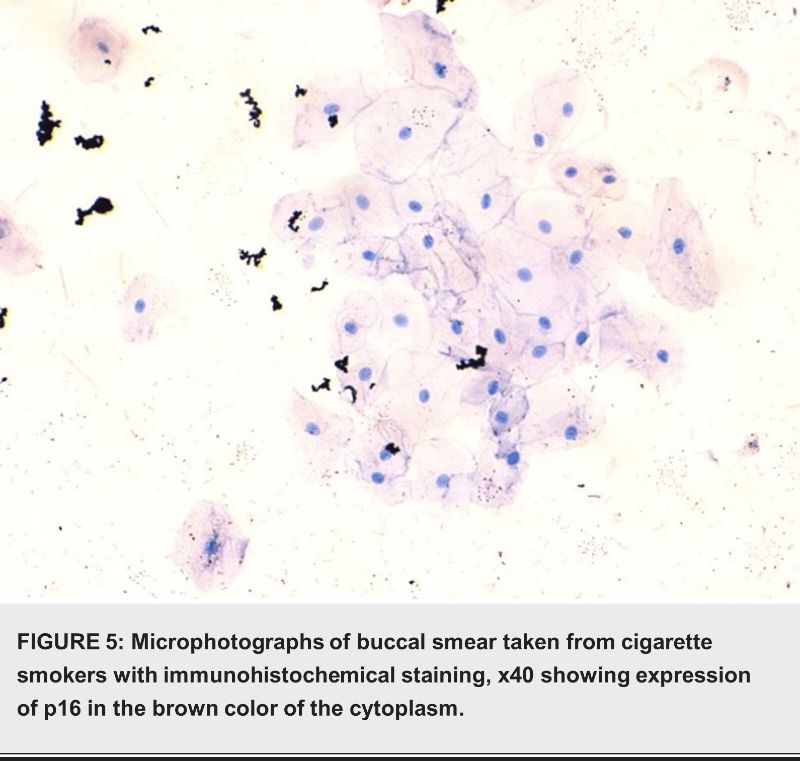 Discussion
Discussion
Oral mucosal alterations are known to be a risk factor for tobacco use, including chewing and smoking. Tobacco contains carcinogens that throw off the balance of antioxidant enzymes that break down and get rid of these harmful chemicals. This could cause sores and abnormal growths in the oral epithelium [15].
This study classified participants according to their gender and age group, as well as the cytopathological alterations identified in cigarette smokers in comparison to individuals who do not smoke. Male smokers were predominantly found in the age category of 31-40, whilst female smokers were most commonly observed in the age group of 18-20. The elevated prevalence of smoking among young adults, namely in the 18-20 age bracket for females, corresponds with previous research that suggests smoking typically begins during adolescence or early adulthood. Commencing tobacco use at an early age heightens the likelihood of engaging in long-term tobacco consumption and experiencing related health complications [16].
The findings showed a clear correlation between smoking and the development of abnormal alterations in the oral epithelium, which can lead to precancerous or malignant diseases. Similar studies, such as the one by Agabeldour et al. in Sudan, found that 47.1% of regular waterpipe users exhibited cytomorphologically unusual changes, compared to none in the control group [17].Smoking and hookah use both generated detectable alterations in the oral mucosa, with smoking having a higher effect, according to a different study that examined cytologic smear samples from the oral mucosa of waterpipe users [18]. A study in Jeddah, Saudi Arabia, revealed that 88.8% of smokers had soft tissue lesions and various oral conditions, although premalignant lesions were less common. This high incidence of oral mucosal soft tissue lesions is likely due to tobacco's irritating effect on oral tissues. The higher number of cytological changes seen in this study could be because of the cigar smokers and toombak users who were there. Both of these things can cause abnormal changes in oral epithelial cells [19].
This study also highlighted the association between cigarette smoking and cytological inflammation in the oral mucosa. Researchers found inflammation in 52% of smokers, compared to 15% of nonsmokers.
Furthermore, there were statistically significant differences in the incidence of bacterial infections (12%) in buccal smears from smokers compared to nonsmokers (5%). These findings are consistent with studies showing that cigarette smoking impairs immune responses, leading to increased inflammation and susceptibility to infections [20].
Contrary to some previous studies, our results did not find a statistically significant difference in p16 expression between smokers and nonsmokers. While studies such as Pandeet et al. in India reported high p16 expression in normal tissues, our findings align with others showing low to undetectable p16 levels in normal human tissues [21]. The variation in results may be due to the different criteria for positivity used in these studies. The increased Ki-67 expression in smokers indicates heightened cellular activity, which is a hallmark of cancerous transformation [22].
The significant association between smoking and increased P16 and Ki-67 expression emphasizes the need for targeted public health interventions to reduce smoking prevalence. Regular screening programs for early detection of oral mucosal changes in smokers could help prevent the progression to malignancy. We need future longitudinal research to establish causality and assess the long-term impact of smoking on oral health.
The study's limitations
The cross-sectional design of this study limits the ability to prove that smoking caused the changes in the cells that were studied. Also, relying on people's self-reported smoking state could lead to information bias. Using biochemical markers to confirm whether or not someone smokes would make the data more reliable.
Conclusions
This study demonstrates that smoking significantly impacts oral cellular health, leading to increased expression of P16 and Ki-67 proteins and a higher incidence of cytological abnormalities. These findings highlight the critical need for smoking cessation programs and regular oral health screenings to mitigate risks. Utilizing P16 and Ki-67 as biomarkers in routine assessments can aid early detection and prevention of oral diseases. Public health interventions should focus on reducing smoking prevalence and raising awareness about its harmful effects. Future research should explore the long-term impact of smoking on oral health and the underlying molecular mechanisms.
Additional Information
Disclosures
Human subjects: Consent was obtained or waived by all participants in this study. Al Rayyan Medical Colleges (AMC) Ethical Committee issued approval HA-03-M-122-045. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.