Oncology and Cancer Screening
OPEN ACCESS | Volume 7 - Issue 1 - 2026
ISSN No: 2994-8746 | Journal DOI: 10.61148/2994-8746/JOCS
Amani Saleh Hadi Saeed
Specialist of clinical oncology and nuclear, National oncology center –Aden. Head of Health Education unit for Arab council of Academic and competencies –branch of Yemen.
*Corresponding author: Amani Saleh Hadi Saeed, Specialist of clinical oncology and nuclear, National oncology center –Aden. Head of Health Education unit for Arab council of Academic and competencies –branch of Yemen.
Received: January 03, 2022
Accepted: January 17, 2022
Published: January 25, 2022
Citation: Amani Saleh Hadi Saeed. (2022) “ Pazopanib for metastatic renal cell carcinoma: A case report”, J Oncology and Cancer Screening, 4(1); DOI: http;//doi.org/001.2022/1.1050.
Copyright: © 2022 Amani Saleh Hadi Saeed. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Malignant renal cell carcinoma (RCC) account for 2-3% of cancer incidence and results in over 100 000 worldwide deaths annually. Metastatic renal cell carcinoma has historically been refractory to cytotoxic and hormonal agent; only interleukin 2 and interferon alpha provide response in a minority of patient. we present case of metastatic renal cell cancer treated with pazopanib with good CR (complete response).
Pazopanib is oral an angiogenesis inhibitor targeting vascular endothelial growth factor receptor, platelet-derived growth factor receptor and c-kit.is approved for the first line treatment of patients with metastatic renal cell carcinoma (mRCC).
Case Overview:
Initial Presentation:
A 35 -year- old female presents with sudden onset lower back pain with hematuria, no h/o DM or hypertension.
Clinical work up:
Treatment and follow-up:
Conclusion: pazopanib provide equivalent anti-tumor effectiveness in treatment of mRCC.
Introduction:
Renal cell carcinoma (RCC) accounts for approximately 1% of all cancers. And incidence of kidney cancer, unlikely other genitourinary malignancies, is rapidly increasing at 2.5% per year.
Metastatic renal cell cancer (RCC) is estimated to have caused 13,010 death in the United States in 2008[1]. Even with early detection and early radical resection ,20-40% of patients experience distance metastasis or recurrence [3]. metastatic renal cell cancer has historically been refractory to cytotoxic and hormonal agents; only interleukin 2 and interferon alpha provide response in minority of patients.
Chemotherapy has consistently been an ineffective from of treatment for RCC [2].
An enhanced understanding of the underlying biology of RCC has led to systemic therapy targeted at the vascular endothelial growth factor VEGF pathway as well as the mammalian target of rapamycin mTOR pathway. Agent blocking these pathway elements have demonstrated robust efficacy, offer new strategic options for patients with metastatic RCC, and have largely replaced cytokines as the standard of care in this disease. Pazopanib is oral an oral angiogenesis inhibiter targeting vascular endothelial growth factor receptor, and c-Kit [4-6]. Sunitinib, pazopanib and five other agents have been approved by Food and Drug Administration for treatment of of clear -cell, metastatic renal cell carcinoma [8,9]. Among the tyrosine kinase inhibiter, pazopanib and sunitinib are first -line treatment options.
a double -blind phase III study of pazopanib 800mg daily versus placebo in a2:1 randomization of treatment -naive and cytokine-pretreated patients with metastatic RCC was recently reported. [7].
Case presentation:
The case we presenting is a-35-year-old woman come with rt flank pain and hematuria suddenly and some lower back discomfort on palpitation .no history of diabetes or hypertension clinical work -up with a CT of chest, abdomen and pelvis (16/7/2018) demonstrated a right -sided 2.5 cm renal mass, partial nephrectomy biopsy, which confirmed clear-cell renal carcinoma (18/9/2018). over, all the surgery was well tolerated and was followed with serial imaging at 3,6 months which was all unremarkable. due to lack of other metastasis lesion recommended fellow -up CTchest+abdomenand pelvic with contrast (13/3/2019) reveled multiple lung tiny nodules measuring about 5mm seen at right upper lobe suspicious pulmonary metastasis otherwise no residual mass or recurrence at site of right renal region, no hepatic or bony metastasis. Sunitinib (50mg x1tablets x daily) was introduced, for three weeks. She showed abnormal liver function marker and developed gastritis with hematemesis, stop treatment with supportive treatment for liver enzymes disturbance. At that time, her laboratory {test result} were all with in normal limits. She remained ECOG {performance status score }0, she was then started on pazopanib, which was well tolerated for two years and she achieved a complete response. When we saw this patient initially, she continued to be good risk given her long duration of being disease free with normal labs and also good performance status, pazopanib would be avery reasonable first -line agent which she had a good response to single -agent TKI {tyrosine kinase inhibitor}. last follow up with PET -scan 12/8/2021:no FDG-avid pulmonary nodules, no evidence of hypermetabolic lesion distinctive for active neoplasia (figure 1).
28/11/2021 liver enzymes increase upper limits, HCV by PCR, Quantitive test:
46 700 IU/ml (reference rang: result more than 10IU/ml is considered positive), patient under regular treatment for hepatitis C, well control till published this article.
Our case is complete response and still alive without any complain or relapse until published this article.
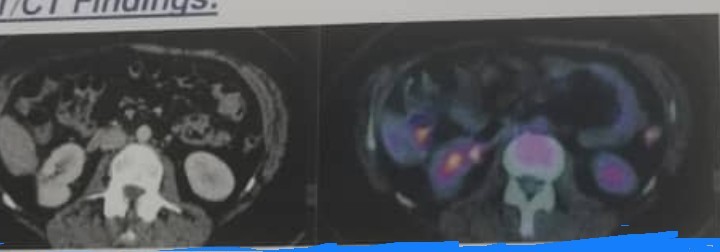
Figure 1: PET/CT finding: evidence of partial right nephrectomy with clear operative bed and no FDG-avid focal lesion, left kidney no FDG-avid focal lesions. Small abdominal lymph nodes with no significant FDG uptake, otherwise no FDG-avid or pathologically enlarged lymph nodes.
Discussion:
Evidence on the effectiveness of pazopanib in the first line treatment of mRCC has been obtained from several clinical trials which have shown a progression -free survival of between 8 and 11 months, either compared to placebo or to sunitinib [10,11]
Most adverse event recorder in the VEG105192 study with pazopanib were grad 1or 2 ;52% of patients had diarrhea; 40% arterial hypertension ;38% hair depigmentation 26% nausea; 22%anorexia; and 21%vomiting [10]. Delea et al. suggested that pazopanib was more cost -effective compared with sunitinib when use as first line treatment among American patients with mRCC [12].as the case treated first with sunitinib for three weeks; not tolerated with sever side effect gastritis and hematemesis ,patient was able to take full dose of pazopanib without experience any side effect for 24months,achieving successively cancer control.TheVEG105192 studies found median PFSvauluesof 9.2 months [11,13].The COMPARZ study revealed no marked different in terms of PFS or OS between sunitinib and pazopanib treatment [11].regarding the quality of life and early tumor shrinkage ,pazopanib showed more favorable result than sunitinib treatment .
Conclusions:
The results confirm that pazopanib is an effective and safe first line targeted treatment in patients with mRCC. pazopanib is an active agent for the treatment of advanced clear cell renal carcinoma, even after sunitinib or bevacizumab.