International Clinical Research and Clinical Trials
OPEN ACCESS | Volume 2 - Issue 1 - 2025
ISSN No: - | Journal DOI: 10.61148/ICRCT
Nesibe AYDOĞDU 1, Mustafa TİMURKAAN1, Esra DALGIÇ 1, Yücel KARACA 2*
1Department of İnternal Medicine, Fethi Sekin Sehir Hastanesi, Elazıg, TURKEY.
1Department of İnternal Medicine, Fethi Sekin Sehir Hastanesi, Elazıg, TURKEY.
1Department of İnternal Medicine, Fethi Sekin Sehir Hastanesi, Elazıg, TURKEY.
2Department of Cardiology, Fethi Sekin Sehir Hastanesi, Elazıg, Turkey.
*Corresponding Author: Yücel KARACA, Department of Cardiology, Fethi Sekin Sehir Hastanesi, Elazıg, Turkey.
Received Date: November 08, 2024
Accepted Date: November 11, 2024
Published Date: December 10, 2024
Citation: AYDOĞDU N, TİMURKAAN M, DALGIÇ E, KARACA Y,. (2024) “Inflammatory Biomarker Ratios as Predictors of Heart Failure in Type 2 Diabetes Mellitus: A Retrospective Analysis.”, International Clinical Research and Clinical Trials, 1(1); DOI: 10.61148/ICRCT/002.
Copyright: © 2024. Yücel KARACA. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Objective:
Systemic inflammation is believed to be related to the pathogenesis of Type 2 diabetes, the formation of complications, and the development of heart failure in the context of diabetes. This study aims to provide insights into whether an increase in inflammatory biomarkers may serve as a predictive factor for impending heart failure in diabetic patients and to present this information to the literature.
Material and Method:
A total of 506 patients diagnosed with diabetes and heart failure (HF) were included in this retrospective study, excluding those with a prior heart failure diagnosis. Diabetic patients were classified according to HbA1c levels. Patients diagnosed with both diabetes and heart failure, with an ejection fraction (EF) below 50 based on echocardiography results, were selected, alongside those receiving diabetes treatment. The ratios of Neutrophil/HDL (NHR), Monocyte/HDL (MHR), Lymphocyte/HDL (LHR), Platelet/HDL (PHR), as well as the systemic immune inflammation index (SII), systemic inflammatory response index (SIRI), Neutrophil/Lymphocyte (NLR), Monocyte/Lymphocyte (MLR), and Neutrophil/Albumin (NAR) were calculated.
Results:
The values of CRP (p<0.001), neutrophils (p=0.014), NHR (p<0.001), MHR (p=0.037), NLR (p<0.001), MLR (p<0.001), NAR (p=0.015), SII (p<0.001), and SIRI (p<0.001) were found to be significantly higher in the DM+HF group compared to the DM group. Additionally, except for the PHR value, all other parameters were observed to be more specific in the DM+HF group compared to the DM group at varying rates.
Conclusion:
To the best of our knowledge, our study is the first in the literature to investigate the relationship between these nine parameters by comparing groups and one another in patients with T2DM and HF. This study demonstrates a strong association between the parameters NHR, MHR, NLR, MLR, NAR, SII, and SIRI with the increased risk of HF in patients with Type 2 DM. Among these parameters, NLR is identified as the most decisive.
Systemic inflammation; HF; HDL; LHR; PHR; NHR; MHR; NLR; MLR; NAR, SII; SIRI
Introductıon
Diabetes Mellitus (DM) is a systemic disease characterized by chronic hyperglycemia, primarily caused by partial or complete insulin deficiency or insulin resistance. The acute metabolic complications of the disease lead to dysfunction in various organs and systems (especially the eyes, kidneys, heart, and blood vessels) over the long term [1]. Insulin resistance and related complications in DM have been linked to inflammatory responses, which are considered the primary molecular mechanism underlying the pathophysiology of the disease [2]. The onset of insulin resistance and DM is associated with the presence of chronic low-grade inflammation. This can trigger other pathophysiological mechanisms, such as β-cell dysfunction and impaired insulin signaling [3]. Heart failure (HF) is a complex clinical syndrome characterized by typical symptoms, such as shortness of breath and fatigue, and is accompanied by evidence of heart dysfunction (e.g., abnormal left ventricular [LV] and/or right ventricular [RV] filling and elevated filling pressures) [4]. HF frequently coexists with diabetes and shares similar metabolic risk factors. The prevalence of DM in HF ranges from 10% to 47%, much higher than in the general population [5-7]. Systemic inflammation is recognized to be associated with the development, progression, and complications of HF [8]. Furthermore, inflammatory markers are considered prognostic factors for poor outcomes in HF. It is acknowledged that chronic low-grade inflammation in HF triggers progression to decompensated heart failure [9]. Recent clinical studies on anti-inflammatory therapies targeting identified inflammatory markers (such as TNF-α and IL-1) have not yielded satisfactory results in preventing this progression [9,10]. Therefore, new inflammatory biomarkers are needed to help identify HF patients who could benefit from anti-inflammatory therapy and reduce their prognostic risks.
In addition to its role in transporting cholesterol, high-density lipoprotein cholesterol (HDL-C) has various protective properties related to infection, inflammation, and thrombosis. Ratios such as neutrophil/HDL (NHR), monocyte/HDL (MHR), lymphocyte/HDL (LHR), platelet/HDL (PHR), the systemic immune-inflammation index (SII), the systemic inflammatory response index (SIRI), neutrophil/lymphocyte ratio (NLR), monocyte/lymphocyte ratio (MLR), and neutrophil/albumin ratio (NAR) have gained considerable attention in recent years as biomarkers. These markers are easy to calculate compared to routine measurements. Blood indicators offer the advantages of being inexpensive and easy to obtain. They can be used in early stages to assess the prognostic risk of cardiovascular diseases in many clinical studies [11-18]. In community-based studies, the risk of death increases in HF patients with DM, both in hospitalized patients and those receiving outpatient care [19, 20]. There are few studies on the early identification and prediction of HF cases in asymptomatic diabetic patients with stable disease. Therefore, better methods for early diagnosis and prediction of HF in patients with DM must be developed, and an effective prognostic factor is crucial for the clinical management of HF in patients with DM.
This study aims to explore whether an increase in inflammatory biomarkers can serve as a predictive factor for impending heart failure in diabetic patients and contribute to the literature on the subject.
Materıals And Methods
For this study, approval was obtained from our Hospital Medical Ethics Committee (2024/10-31). Written informed consent forms were signed by all patients. A total of 506 patients were included in the study, comprising 249 patients diagnosed with DM+HF who were hospitalized in the cardiology clinic of Fethi Sekin City Hospital between 2022 and 2024, and 257 diabetic patients without a diagnosis of heart failure who were hospitalized in the internal medicine clinic. Diabetic patients were classified based on their HbA1c levels. Among patients diagnosed with both diabetes and heart failure, those with an ejection fraction (EF) below 50 and receiving diabetes treatment based on their echocardiography results were selected. The medical records of all patients were retrospectively reviewed from the hospital system, including data on gender, age, HbA1c, EF (ejection fraction), biochemical blood lipid profiles, AST, ALT, albumin, urea, creatinine, and hemogram results. The neutrophil/HDL (NHR), monocyte/HDL (MHR), lymphocyte/HDL (LHR), platelet/HDL (PHR), systemic immune-inflammation index (SII), systemic inflammatory response index (SIRI), neutrophil/lymphocyte ratio (NLR), monocyte/lymphocyte ratio (MLR), and neutrophil/albumin ratio (NAR) were calculated for all patients.
Inclusion criteria:
1) Aged 18 or older,
2) A history of previously diagnosed diabetes,
3) Diagnosis of heart failure with an ejection fraction below 50 as assessed by echocardiography along with diabetes.
Exclusion criteria:
1) Under the age of 18,
2) Malignant disease,
3) Receiving glucocorticoid treatment,
4) Being pregnant,
5) Having a hematologic disease,
6) Having immunodeficiency for any reason.
Statıstıcal Analysıs
The analyses were performed using the SPSS (Statistical Package for Social Sciences; SPSS Inc., Chicago, IL) version 22 software. Descriptive data were presented as n and % values for categorical variables, and as median and interquartile range (25th-75th percentile values) for continuous variables. The Chi-square test (Pearson Chi-square) was used to compare categorical variables between groups. The Kolmogorov-Smirnov test was used to assess the normality of the distribution of continuous variables. The Mann-Whitney U test was used to compare two groups. Receiver Operating Characteristic (ROC) curves were plotted to measure the value of various parameters in predicting the diagnosis of DM+HF. A p-value of less than 0.05 was considered statistically significant.
Results
A total of 506 patients were included in the study, with 249 patients in the DM+HF group and 257 patients in the DM-only group. There was no significant difference in age between the groups (p=0.251). Of the DM+HF group, 42.6% were female and 57.4% were male, while in the DM group, 49.8% were female and 50.2% were male. There was no significant difference in gender distribution between the groups (p=0.103). The EF (p<0.001), HbA1c (p<0.001), glucose (p=0.028), HDL (p<0.001), LDL (p=0.007), triglyceride (p=0.015), albumin (p=0.001), platelet (p<0.001), lymphocyte (p<0.001), and LHR (p=0.001) levels in the DM+HF group were significantly lower than those in the DM group. On the other hand, urea (p<0.001), creatinine (p<0.001), AST (p<0.001), CRP (p<0.001), neutrophil (p=0.014), NHR (p<0.001), MHR (p=0.037), NLR (p<0.001), MLR (p<0.001), NAR (p=0.015), SII (p<0.001), and SIRI (p<0.001) levels were found to be significantly higher in the DM+HF group (Table 1).
|
|
DM+HF (n=249) |
DM (n=257) |
p* |
|
|
Median (IQR) |
Median (IQR) |
|
|
Age |
70.00 (63.00-77.00) |
68.00 (59.00-79.00) |
0.251 |
|
Gender (F/M) |
106/143 |
128/129 |
0.103** |
|
EF (%) |
35.00 (30.00-40.00) |
55.00 (55.00-60.00) |
<0.001 |
|
HbA1c (%) |
8.70 (7.10-10.20) |
9.60 (7.90-11.70) |
<0.001 |
|
Glucose (mg/dL) |
186.00 (141.00-246.00) |
196.00 (137.00-322.00) |
0.028 |
|
Urea (mg/dL) |
52.00 (37.00-84.00) |
36.60 (28.00-48.00) |
<0.001 |
|
Creatinine (mg/dL) |
1.02 (0.80-1.40) |
0.79 (0.63-0.98) |
<0.001 |
|
AST (U/L) |
21.00 (17.00-33.00) |
18.00 (15.00-24.00) |
<0.001 |
|
ALT (U/L) |
19.00 (13.00-32.00) |
18.00 (13.00-26.00) |
0.141 |
|
CRP (mg/dL) |
13.00 (5.96-29.00) |
7.62 (3.79-21.10) |
<0.001 |
|
HDL (mg/dL) |
41.00 (34.00-48.50) |
47.00 (39.00-55.00) |
<0.001 |
|
LDL (mg/dL) |
88.00 (66.00-122.00) |
102.00 (78.00-125.00) |
0.007 |
|
Triglyceride (mg/dL) |
128.00 (92.00-184.00) |
145.50 (105.00-201.00) |
0.015 |
|
Albumin (mg/g) |
37.00 (34.00-40.00) |
38.00 (34.00-41.00) |
0.001 |
|
Hb (g/dL) |
13.00 (11.30-14.60) |
13.20 (11.70-14.50) |
0.313 |
|
Platelet (109/L) |
233.00 (190.00-285.00) |
256.00 (214.00-309.00) |
<0.001 |
|
Neutrophil (109/L) |
5.49 (4.30-6.96) |
4.99 (3.79-6.61) |
0.014 |
|
Lymphocyte (109/L) |
1.65 (1.14-2.20) |
2.22 (1.56-3.01) |
<0.001 |
|
Monocyte (109/L) |
0.67 (0.51-0.80) |
0.61 (0.47-0.86) |
0.345 |
|
NHR |
0.13 (0.10-0.19) |
0.11 (0.07-0.16) |
<0.001 |
|
MHR |
0.02 (0.01-0.02) |
0.01 (0.01-0.02) |
0.037 |
|
LHR |
0.04 (0.03-0.06) |
0.05 (0.03-0.07) |
0.001 |
|
PHR |
5.70 (4.39-7.56) |
5.68 (4.21-7.18) |
0.329 |
|
NLR |
3.33 (2.24-5.05) |
2.12 (1.47-3.35) |
<0.001 |
|
MLR |
0.39 (0.28-0.60) |
0.28 (0.20-0.43) |
<0.001 |
|
NAR |
0.15 (0.12-0.21) |
0.13 (0.10-0.19) |
0.015 |
|
SII |
762.66 (476.67-1177.38) |
537.52 (356.79-882.70) |
<0.001 |
|
SIRI |
2.04 (1.40-3.58) |
1.46 (0.85-2.92) |
<0.001 |
*p-values are based on the Mann-Whitney U test. **Chi-square analysis was applied.
Table 1: Comparison of all characteristics of the groups
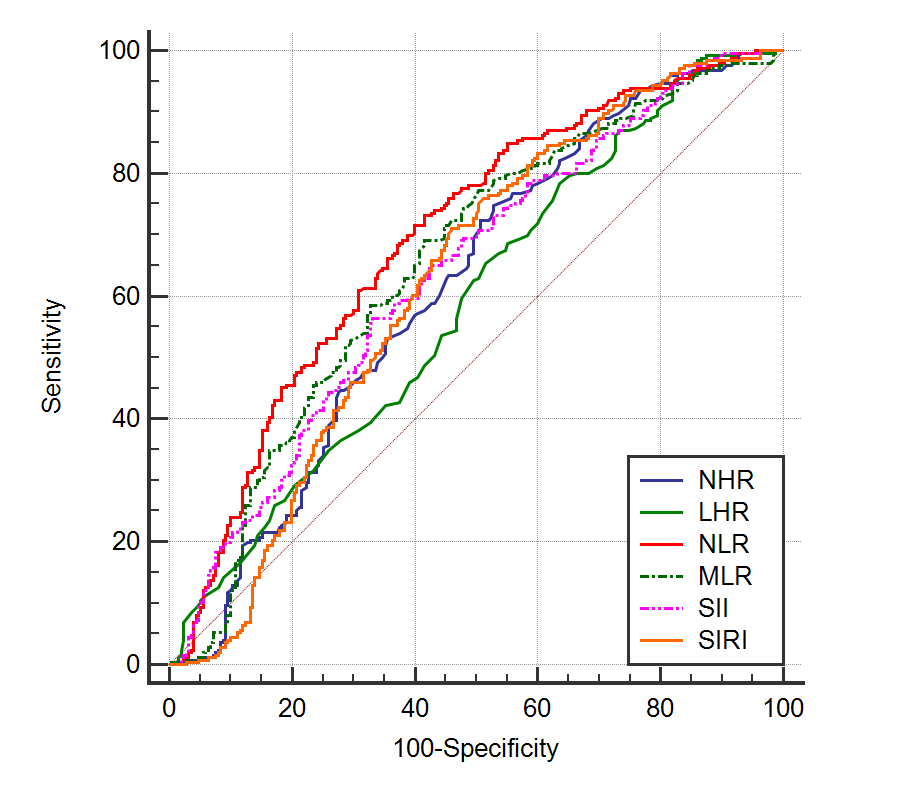
ROC analysis was conducted to investigate the ability of various values to predict DM+HF disease, and the cut-off values were determined. When the cut-off value for NHR was set at 0.1, the sensitivity was 74.8% and the specificity was 47.2%, indicating that it is a good predictor. For MHR, a cut-off value of 0.013 was found to have a sensitivity of 65% and a specificity of 49.6%, showing it to be a good predictor. For LHR, a cut-off value of 0.059 yielded a sensitivity of 78.5% and a specificity of 36.4%, proving it to be a good predictor. PHR, with a cut-off value of 6.682, had a sensitivity of 37.8% and a specificity of 69.6%, indicating it is a poor predictor. NLR, with a cut-off value of 2.381, exhibited a sensitivity of 72.9% and a specificity of 57.2%, demonstrating it as a good predictor. For MLR, a cut-off value of 0.306 was associated with a sensitivity of 69.2% and a specificity of 57.2%, indicating it is a good predictor. When the cut-off value for NAR was set at 0.118, the sensitivity was 72% and the specificity was 42.5%, making it a good predictor. SII, with a cut-off value of 704.987, had a sensitivity of 56.3% and a specificity of 65.4%, showing it is a good predictor. For SIRI, a cut-off value of 1.514 resulted in a sensitivity of 71.3% and a specificity of 52.9%, proving it to be a good predictor (Table 2 and Figure 1).
Table 2. Specificity and Sensitivity of Measured Parameters in Identifying DM+HF Disease
|
|
Area |
p |
95% Confidence Interval |
Sensitivity |
Specificity |
PP |
NP |
|
|
Lower Limit |
Upper Limit |
|||||||
|
NHR>0,1 |
0,616 |
<0,001 |
0,571 |
0,659 |
74,8 |
47,2 |
58,2 |
65,6 |
|
MHR>0,013 |
0,553 |
0,042 |
0,508 |
0,598 |
65 |
49,6 |
55,9 |
59 |
|
LHR≤0,059 |
0,588 |
<0,001 |
0,543 |
0,631 |
78,5 |
36,4 |
54,8 |
63,2 |
|
PHR>6,682 |
0,525 |
0,345 |
0,480 |
0,569 |
37,8 |
69,6 |
55 |
53,2 |
|
NLR>2,381 |
0,683 |
<0,001 |
0,641 |
0,724 |
72,9 |
57,2 |
62,1 |
68,7 |
|
MLR>0,306 |
0,646 |
<0,001 |
0,603 |
0,688 |
69,2 |
57,2 |
60,9 |
65,9 |
|
NAR>0,118 |
0,564 |
0,016 |
0,518 |
0,609 |
72 |
42,5 |
55,9 |
59 |
|
SII>704,987 |
0,625 |
<0,001 |
0,581 |
0,668 |
56,3 |
65,4 |
61,0 |
60,9 |
|
SIRI>1,514 |
0,613 |
<0,001 |
0,569 |
0,656 |
71,3 |
52,9 |
59,3 |
65,7 |
Figure 1: ROC Curve of NHR, LHR, NLR, MLR, SII, and SIRI Values in Identifying DM+HF Disease.
Dıscussıon
Recent significant studies have highlighted the widespread role of monocytic immunity in diabeticogenesis, linking it to islet cell inflammation, beta cell dysfunction, and insulin resistance [21, 22]. Additionally, it has been shown that HDL-C metabolism adversely affects monocytosis and weakens monocytic metabolism [23]. Furthermore, HDL deficiency is commonly observed in environments predisposed to diabetes, primarily due to insulin resistance and lipid disorders [24, 25]. Thus, the use of MHR as a biomarker to monitor inflammatory imbalance prior to the onset of diabetes is suggested [26].
In diabetes mellitus (DM), metabolic disturbances (hyperglycemia, hyperinsulinemia, obesity, chronic inflammation) lead to oxidative stress, autonomic neuropathy, and impaired calcium homeostasis. This ongoing process contributes to endothelial dysfunction, atherosclerosis, and ultimately heart failure [27]. Heart failure (HF) is frequently associated with diabetes, as both share similar metabolic risk factors. Community-based studies have indicated an increased risk of mortality in patients with HF accompanying DM, both in hospitalized and outpatient settings [19, 28]. Therefore, there is an ongoing effort to identify new biomarkers that can aid clinicians in taking preventive measures and achieving early diagnosis for the impending risk of heart failure in diabetic patients.
Previous studies have demonstrated elevated levels of C-reactive protein (CRP), interleukin (IL)-1, tumor necrosis factor-alpha (TNF-α), and inflammatory cytokines in diabetic patients [29]. These inflammatory changes play a direct role in the pathogenesis of complications such as nephropathy, neuropathy, and atherosclerosis [30]. In a study by Hansson GK, the measurement of inflammatory markers such as CRP and pro-inflammatory cytokines (e.g., interleukin-6) was shown to provide insights into coronary artery disease and inflammatory conditions [31]. Another study by Dieden A. et al. highlighted a significant association between elevated levels of Galectin-4 and diabetes as well as high glucose levels among heart failure patients [32]. The primary aim of these studies has been to propose inflammatory parameters as biomarkers that could indicate the risk of heart failure in patients diagnosed with diabetes mellitus. However, due to the high cost of the materials used, the quest for new biomarkers that are easier to implement and more cost-effective continues.
Recently, numerous studies have been conducted on NHR, MHR, LHR, PHR, NLR, MLR, NAR, SII, and SIRI due to their ease of use, demonstrating that each has significant clinical value. However, there has yet to be a comprehensive study examining the relationship between these inflammatory parameters and T2DM–HF collectively. One study indicated that elevated PHR levels associated with type 2 diabetes mellitus (T2DM) are linked to adverse effects in coronary artery disease and that PHR levels are valuable in identifying high-risk individuals for heart failure [33]. According to our results, PHR levels did not show significance compared to other parameters based on ROC analysis cut-off values. This discrepancy may stem from our selected patient group being clearly aligned with heart failure criteria. This difference is plausible for us, as we believe that platelet function and levels might vary due to the use of various or high doses of anticoagulants in heart failure patients. In this case, we recommend re-evaluating this parameter in patients receiving anticoagulants.
One study demonstrated that NLR is an important factor in identifying macrovascular disease in diabetic individuals [34]. Another study argued that elevated MHR in patients with diabetic ischemic heart failure is an independent predictor of all-cause mortality and myocardial infarction [35]. Additionally, a study showed strong associations between SII in diabetic patients and all-cause and cardiovascular mortality [36]. In another study, it was indicated that SII and SIRI inflammatory markers did not create a significant difference in groups of patients with diabetes and prediabetic coronary syndrome [37]. However, according to our results, we identified a correlation between SII and SIRI and cardiovascular risk in all patients. We believe that these varying results regarding SII and SIRI across studies likely do not stem from individual inflammatory responses but rather from genetic, racial, and regional factors. This is because inflammation plays a significant role in the pathogenesis of both conditions (T2DM and HF).
A study on heart failure demonstrated that the NAR marker has a high ability to predict cardiovascular mortality when compared to albumin or neutrophil percentage alone [38]. However, there is no existing study that predicts heart failure in diabetic patients using NAR. Our study is the first in this regard. Although the NAR value was significant according to our results, we prefer not to include this parameter among predictive parameters, contrary to Wang X et al. This preference is due to the high likelihood of hypoalbuminemia in heart failure patients. Despite our results being significant, this discrepancy between the two groups in our study raises doubts about their predictive value. Thus, NHR, LHR, NLR, MLR, SII, and SIRI can be used more objectively as predictive values. In our comparison, we identified LHR as having the highest sensitivity and SII as having the highest specificity. When we compared these six parameters among themselves and examined the ROC curve we obtained, NLR emerged as the predictive parameter that was both sensitive and specific overall.
Conclusion
In this retrospective cross-sectional study, we investigated the relationship between T2DM and HF by comparing these nine parameters across groups and with each other for the first time. The study demonstrated that NHR, MHR, NLR, MLR, NAR, SII, and SIRI parameters are strongly associated with an increased risk of heart failure in patients with type 2 diabetes mellitus. Preventing inflammation from the outset may play a key role in the pathophysiology of both diseases and could serve as a way to predict or prevent the onset of the disease. When inflammatory parameters are examined in detail, they may act as early indicators for the development of heart failure in patients with T2DM. Among these parameters, NLR stands out as the most predictive marker.
Conflict of Interest: None
Data availability: All needed data can be obtained from corresponding author
Funding: No
This study was approved by the local ethics committee in accordance with the International Code of Ethics and the Declaration of Helsinki.