Ophthalmology and Vision Care
OPEN ACCESS | Volume 4 - Issue 1 - 2025
ISSN No: 2836-2853 | Journal DOI: 10.61148/2836-2853/OVC
Moyseyenko N. M
Associate Professor of Otorhinolaryngology and Ophthalmology with a course of head and neck surgery Ivano-Frankivsk National Medical University, Ivano-Frankivsk, Ukraine
*Corresponding author: Moyseyenko N. M, Associate Professor of Otorhinolaryngology and Ophthalmology with a course of head and neck surgery Ivano-Frankivsk National Medical University, Ivano-Frankivsk, Ukraine
Received date: February 08, 2021
Accepted date: February 16, 2021
published date: February 18, 2021
Citation: Moyseyenko N. M. “The Optic Nerve’s Perfusion Processes and Axonal Transport.’’. Ophthalmology and Vision Care, 1(1); DOI: http;//doi.org/03.2021/1.1002.
Copyright: © 2021 Moyseyenko N. M. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly Cited.
The optic nerve has a number of devices that regulate the function of axons and provide the energy needs of neurons. Although significant progress has been made in their understanding, the treatment of conditions that damage axons and lead to the death of GCS remains limited.
The purpose of the study: To investigate the patterns of perfusion processes and axonal transport of the optic nerve according to the literature.
Research methods: An analysis of 58 sources of literature.
According to the literature, it is known that there are two main regulators of energy and perfusion processes in the optic nerve, which act on the anti- and retrograde pathways, which provide the movement of both nerve impulses (essentially a solution of electrolytes) and elements of the cytoskeleton and neurotransmitters, through which the retina-cerebral and cerebra-retinal effects are carried out.
An important role in the regulation of perfusion and axonal transport is played by astrocytes, which under physiological conditions provide trophism of neurons, axons, as well as the exchange of neurotransmitters and other active conditions.
Conclusion: Thus, the study of processes that provide optic nerve perfusion, as well as the mechanisms of their violation is important for the study of optic neuropathies of various origins.
Introduction
The optic nerve has a number of devices that regulate the function of axons and provide the energy needs of neurons. Fluid perfusion in the optic nerve is of interest to scientists in many medical fields. It is believed that axonal transport provides the propagation of excitation from the ganglion cells of the retina (ҐKS) to the subcortical pathways, and on the way back - contribute to the imperative regulation of the brain processes of the visual system through the spread of neurotransmitters. That is why the violation of such interaction is the basis for both the development of atrophy and regeneration of the optic nerve.
Although significant progress has been made in their understanding, the treatment of conditions that damage axons and lead to the death of ҐKS remains limited
The purpose of the study: To investigate the patterns of perfusion processes and axonal transport of the optic nerve according to the literature.
Research methods: An analysis of 56 sources of literature.
Result: According to the literature, the head of the optic nerve provides a balance of substances for the axons of ҐKS. Almost 50% of the cells of the optic nerve head are glia. Astrocytes play a crucial role in maintaining axonal homeostasis. They are interconnected by spaces and act as a syncytium to buffer changes in the extracellular environment of the axon [ 2]. Behind the sclera through the lattice axons wrapped in oligodendrocytes enter the retrolaminar part of the optic nerve.
Axons ҐKS provide communication between the eye and the brain. An axonal pathway is formed that integrates nerve impulses from the entire retina and converts them into active potential. This activity has a high energy dependence, and therefore is dependent on metabolic and ischemic processes that occur in the retina and along the nerve. Myelination of fibers allows to conduct salts during carrying out the active potential reducing energy requirement for a cell.
The relative sizes of the axon and GCS indicate their high metabolic needs. While the cell body is the main site for energy production and protein synthesis, the axon itself can provide some synthetic functions. The metabolic balance of an axon is provided in two ways. First, due to mitochondria and metabolism to meet the energy needs of the axon, and secondly due to the blood supply to the optic nerve.
Mitochondria is the site of oxidative generation of adenosine triphosphate in the (ATP) as and accumulate in the area of enhanced energy needs [ 3]. Mitochondria do not have a fixed place within the axon, they can move to regions where demand is higher. During development, mitochondria move to regions where axonal growth occurs and the cone expands. When growth is complete, mitochondria are redistributed throughout the neuron [4]. Mitochondria are crucial for the survival of ҐKS in diseases such as Lieber’s optic neuropathy or autosomal dominant neuropathy, where mitochondrial dysfunction occurs retinal cell death and persistent vision loss [5].
According to the metabolic requirements of axons, goal and VC and optic nerve multiway and on vascular plexus and thin capillaries. Anastomosis insufficiency is thought to underlie conditions such as anterior ischemic optic neuropathy, in which high visual function may persist due to the peculiarities of blood circulation in the optic nerve basin, and compensation by choroidal arterioles [ 6].
Ensuring a stable supply of oxygen is essential to maintain the metabolic needs of the axons of the optic nerve head. This is done under conditions of fluctuations in intraocular (IOP) and systemic blood pressure. The property of the choroid to balance pressure fluctuations while maintaining optic nerve perfusion is shown. However, the dynamic range for this autoregulation is limited, with IOP above 45 mm. rt. Art. perfusion decreases sharply. D feisty autoregulation can occur regionally within the optic nerve head. It is known that oxygen levels in the optic nerve sashayed be constant during periods of hypoxia -induced systemic the hypotensive you [ 7]. Autoregulation ensures the preservation of perfusion in excess of blood pressure, preventing ischemia of the optic nerve head. Deep nocturnal hypotension and decreased perfusion of the optic nerve head, resulting from systemic shock leads to the development of glaucomatous excavation [8]. Multiple studies have shown that there are no precapillary sphincters within the capillary bed of the optic nerve head. At the same time, capillary pericytes have contractile properties, which allows you to change the pressure inside the capillary bed and thus regulate stable perfusion. It is known that inside the head of the optic nerve pericytes react by narrowing with a decrease in oxygen or acidity [9].
Adenosine is a strong relaxing factor for pericytes, which provides increased blood flow in areas with high metabolic demand and high ATP intake [10]. Such mechanisms regulate the balance of potassium ions and carbon dioxide. Angiotensin II regulates the sensitivity to carbon dioxide inside the head of the optic nerve [11].
Endothelial cells play an important role in controlling the tone of the vessels of the optic nerve head through the production of nitric oxide, increasing permeability to blood. Violation of this mechanism plays a pathogenetic role in the formation of glaucoma, diabetic retinopathy and compression neuropathy [12]. Regulation is also carried out through the production of endothelin 1, which affects the hemodynamics of DZN, and its disorders contribute to the death of GCS. In particular, in ischemic neuropathy, an increase in the concentration of endothelin-1 in plasma has been determined [13, 14].
In addition, endothelin receptors are found in astrocytes, which has a control over their proliferation and a direct effect on axon function. Elevated endothelin levels reduce anterograde mitochondrial transport, thereby impairing local ATP supply [15, 16].
In addition to its primary function in conducting action potentials, the axon allows the body of the neuron to make metabolic contact through its endings with targets in the lateral cranked nucleus and upper humps in the quadrupedal body. These processes are important for the survival of neurons in disease.
This interaction between the structures ensures the transport of molecules, vesicles and organelles in both directions. The anterograde pathway is ascending, directed from the body ҐKS to the brain. Retrograde path - descending, from the lateral geniculate body. Disruption of this transport process as a result of focal damage to the optic nerve, or damage to the population of cells in contact with the nerve ending of the axon, can lead to the death of ҐKS. In experimental glaucoma, for example, a decrease in the level of neurotoxic factor (BDNF), which is retrogradely transported to the body ҐKS leads to their increased death. On the other hand, exogenous BDNF protection protects these cells from such damage [17, 18].
Axonal (anterograde and retrograde) transport of molecules, subcellular organelles and metabolic products occurs along the optic nerve, is energy-dependent and requires a high concentration of oxygen, and therefore is disrupted by ischemic, inflammatory and compression processed
Anterograde axonal transport system is divided into slow, medium and high-speed parts. Rapid transport is trans synoptically through vesicles located at the presynaptic ends of the axon. In this way, proteins are transported, and the process is potentiated by enzymes called neurotransmitters.
Slow antirad transport provides the movement of proteins synthesized in the nerve cell, which include components of the cytoskeleton and protein complexes that are delivered to the axon and its ends. Slow anterograde transport is divided into two components: slow A transport in relation to the distribution of triplet proteins of neurofilaments, such as tubulin and spectrum, as well as tau proteins; slow B transport is faster and involves the transfer of microfilaments, actin and proteins of supramolecular complexes of the cytosolic matrix [ 19 , 20 ].
High binding affinity leads to continuous and faster transport. Under other conditions, it is mediated by components of low affinity, and therefore is slower. Microtubules themselves are highly polarized structures of alpha and beta-tubulin. Under certain conditions, they are grouped. The "minus" end is close to the cell body and the "positive" end is close to the end of the axon, usually more unstable and associated with the growing end of the axon.
Molecular control for anterograde transport is provided by kinesin molecules belonging to the family of specialized motor proteins. Kinesins have a motor domain that hydrolyzes ATP to move microtubules down the axon. Kinesins are synthesized in the cell body and stored in soluble form in the cytoplasm. Kinesin control is activated by binding to the "cargo" molecule, which is then traced along the microtubules of the axon to the end (Fig. 1).
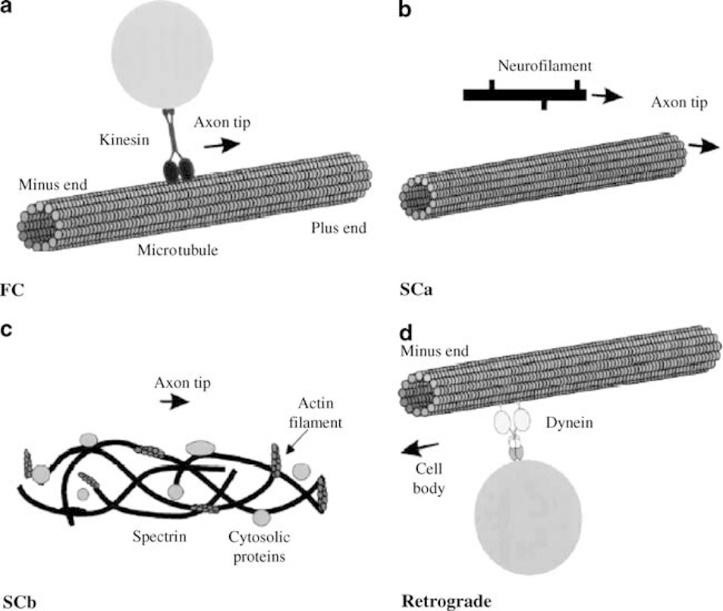
Figure 1: Scheme of axonal transport in the optic nerve
Designation: fast transport (Fc), due to the connections between the kinesin complex of the molecule and the axon microtubule for fast antegrade axoplasmic transport from the negative end (Minus end) to the positive end (Plus end)). Slow transport (Scab) through the microtubule (b) or (Sc.B.) using spectrum (Actin filament) and cytosolic proteins (crosslinked proteins) (c). Retrograde transport using the driver molecule dynein (Dynein), which is carried out through the microtubule of the axon to the cell body (Cell body) (d) [ 21].
There are different types of kinesin that provide different modes of transport. Axonal myosin is involved in the delivery of the cargo molecule at the end of the axon and can also modulate the rate of anterograde axonal transport [22, 23].
Retrograde transport is classified as fast. It is controlled by mediators contained in endosomes and lysosomes and absorbed by membrane receptors. The molecules are transported towards the body of neurons. As a mediator, dynein is used [ 24 ], which is first synthesized in the cell body, and then spreads along the axon to the "positive end" through both anti- and retrograde transport systems and activates retrograde transport towards the neuron body [ 25 ]. For slow transport, the actin-spectrum system within the axon is also used more often than through microtubules.
Under normal conditions, astrocytes establish contact with retinal neurons, ensuring the metabolic stability of nerve tissue [2 6]. Physiological studies have identified important functions that these cells perform in the optic nerve and other parts of the CNS. Thus, they are responsible for storing glycogen by supplying glucose to neurons. R halutz the level of extracellular potassium, important in the regulation and metabolism of neurotransmitters such as GABA. They help in the removal of CO2 from the retina. Contribute to the maintenance of water homeostasis in the retina [27, 28].
Astrocytes (Fig. 2) also provides the barrier function of the retina [29, 30]. At the level of the optic nerve, astrocytes are responsible for the secretion by axons of BDNF and associated neurotrophies produced by ҐKS. They also interact with specific transmembrane receptors of cells, which decrease in size after ligand binding and are included in vesicles for retrograde transport into the body of the neuron [[31].
Obstruction of rapid transport leads to a violation of the integrity of the distal axon. Thus, in glaucoma in violation of axonal transport is considered the basis of the pathogenesis of axonal damage GKS as a result of increased IOP. Under such conditions, the axonal cytoskeleton is disturbed, there is disengagement and edema of neurofilaments. Thus, there is a mechanical blocking of axonal transport at the earliest time [32]. Loss ҐKS under such conditions as disorders accompanied by active transport and passive diffusion in a klykayuchy Valerenes degenerates distal axons of place lesions [ 33, 34 ].
Thus, it has been shown that the exchange of molecules and organelles within the neural network makes neurons disrupted in many diseases [ 35].
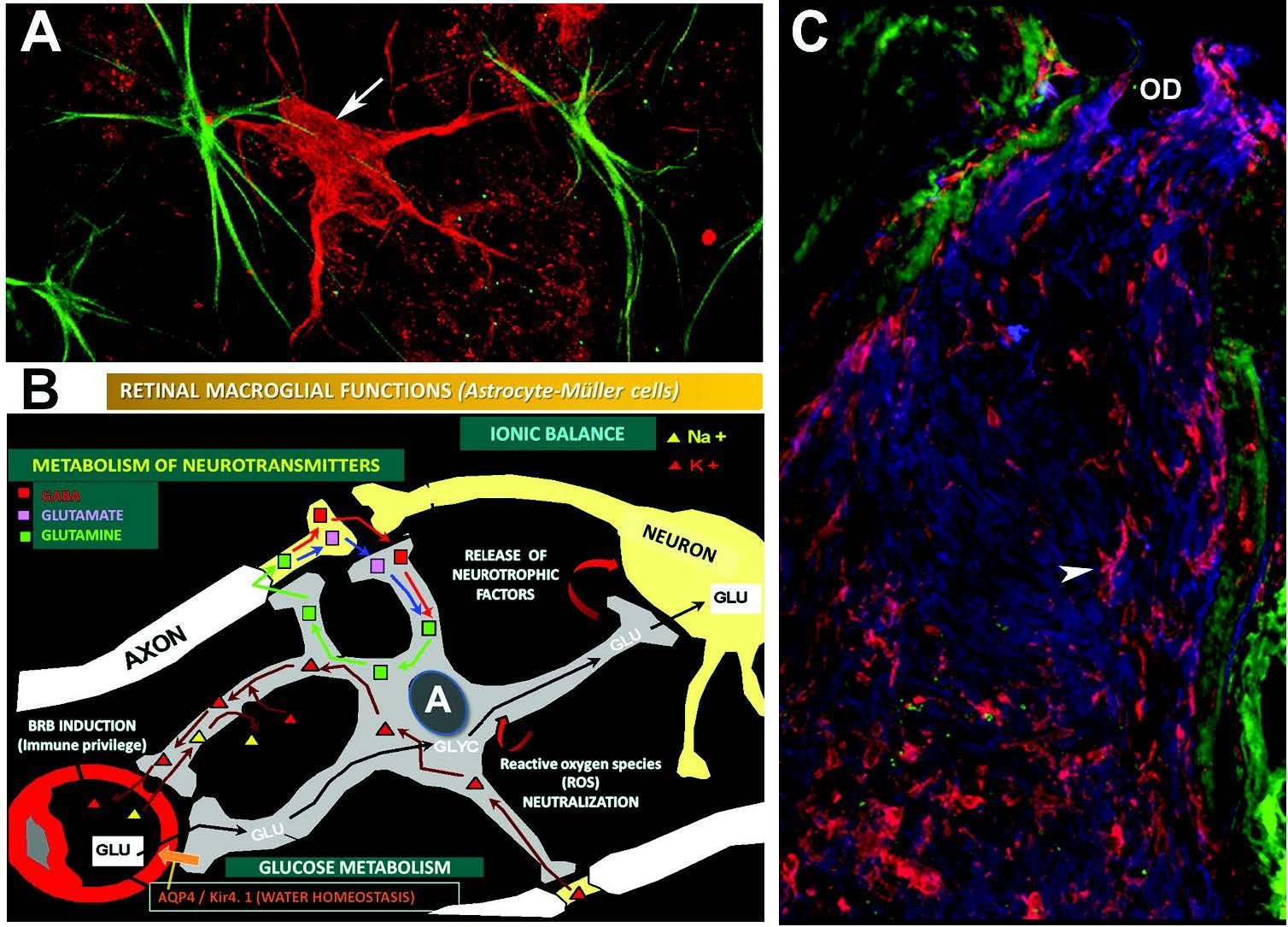
Figure 2: Organization of axonal transport
Symbols: (A) Axton blockage in experimental glaucoma: СCS indicated by red arrow, with neurofilaments blocked in the cytoplasm, astrocytes indicated by green arrow. Retinal immunofluorescence. (B) Scheme of astrocyte function in the optic nerve [56]. (C) The microglia are indicated by an arrow. Optic nerve immunofluorescence [36]
The speed of slow axonal transport involves the transport of molecules over long distances. Some axons take several years for the molecules to reach their nerve endings. Therefore, axons have the property of their own synthesis [ 37] due to peri axonal ribosomes [38].
An important component of fluid perfusion in the optic nerve is the pressure gradient within the lattice plate, which is created by the difference between ocular (IOP), cranial, and orbital pressures [39, 40]. Violation of the dynamics of cerebrospinal fluid can cause normotensive in hakim in [41, 42].
It is known that cerebrospinal fluid circulates in the subarachnoid space of the optic nerve, as well as in the perivascular spaces of blood vessels that penetrate the nerve in the apex, and between the processes of astrocytes. Under such conditions, the cerebrospinal fluid (CSF) that surrounds the nerve provides buoyancy, nutrient delivery and cleanses of breakdown products [ 43]. On the other hand, for the optic nerve, the CSF surrounding nerve fibers is a source of proteins and peptides, such as neurotrophic factor and growth factor [44, 45].
In the brain, a glymphatic pathway is described in which the CSF flows into and out of the parenchyma through the spaces formed between the blood vessels and the palatine layer of the astrocytic benefit. The lymphatic pathway consists of perivascular inflow of CSF into the brain parenchyma, purification of fluid and solutes in the extracellular space.
The course of cerebrospinal fluid in the optic nerve may be similar to the glymphatic system of the brain, which consists of par arterial inflow into the brain parenchyma and perivenous outflow of intercellular fluid and solutes [ 4 6]. The glymphatic system cleanses the brain of harmful metabolites through the water channel and aquaporin-4 at the astrocytic end. Such a perivascular pathway also facilitates the penetration of CSF into the optic nerve [47].
However, differences in the vascular anatomy of the optic nerve compared to the brain call into question whether there is a glymphatic system in the optic nerve. It is known that the retrolaminar optic nerve of the mouse consists only of capillaries with intussusception of the soft membrane, while the brain has large penetrating arteries with thick walls surrounded by both soft shell and glia [48]. It is possible that the flow of CSF through the optic nerve along the perivascular pathways occurs on the principle of simple diffusion or a combination of diffusion and pulsation [49].
Aquaporin-4 is an integral membrane protein found on the astrocytic endophyte (growth stimulator), which promotes the permeability of water to cell membranes and the perivascular space of the optic nerve. Aquaporin-4 thus facilitates the transport of cerebrospinal fluid and perivascular fluid [[50]. In optic neuromyelitis, the formation of anti-Aquaporin-4 antibodies has been detected, which block the flow of perivascular fluid, which leads to demyelination of nerve fibers [51, 52].
Sometimes neurodegenerative diseases occur due to an imbalance between the production and purification of neurotoxic substances in the optic nerve. Under such conditions, the accumulation of amyloid substances has been described, for example in glaucoma [53, 54].
Glum, which covers the gratings around the disk, is also important for CSF penetration. Thus, the CSF cleanses the laminar and retrolaminar parts of the optic nerve head of neurotoxins. It is disorders of current regulation of CSF and glial water and solutions that serve as a pathogenetic element of glaucoma [55] and traumatic fragmentation of the optic nerve [56].
The enzyme Rho kinase, a low IOP inhibitor that enhances intraocular outflow, has also been described. Rho kinase activation has a beneficial effect on neurons in traumatic injuries [ 57, 58].
Conclusion: Thus, according to the literature, we know that Mr. enteritis I and redistribution of ATP serving a count done in mitochondria Yahya who migrate to areas of greatest growth and activity. The redistribution of nutrients within the axons and in the perineural space is ensured by capillary hemodynamics, which also has a self-regulatory system, due to active substances such as adenosine and endothelin 1. This is due to the survival of ҐKS and their axons in critical conditions for the general organism.
There are two main regulators of energy and perfusion processes in the optic nerve, which act on the anti- and retrograde pathways, which provide the movement of both the nerve impulse (which is essentially dissolved electrolytes) and elements of the cytoskeleton and neurotransmitters through which retina-cerebral and cerebra-retinal effects.
Rapid antirad transport is carried out transapically, through vesicles and by means of mediators (neurotransmitters). Slow antirad transport provides the movement of protein synthesized in the nerve cell, cytoskeleton components and protein complexes. It is carried out due to proteins of neurofilaments and supramolecular complexes of the cytosolic matrix. Retrograde transport - the mediator dynein is used.
An important role in the regulation of perfusion and axonal transport is played by astrocytes, which under physiological conditions provide trophism of neurons, axons, as well as the exchange of neurotransmitters and other active conditions.
Conclusion. Thus, the study of processes that provide perfusion of the optic nerve, as well as the pathogenetic mechanisms of their violation is important for the study of forms of optic neuropathy