Journal of Veterinary Medicine And Science
OPEN ACCESS | Volume 3 - Issue 1 - 2026
ISSN No: 3065-7075 | Journal DOI: 10.61148/3065-7075/JVMS
Tahmid Eshad Rupai1, Ummay Ayman1, Latifa Akter1, Md. Zahirul Islam Khan2, Md. Abdul Awal1, Md. Najmul Hasan Parvez3, Ziaul Haque1*
1Department of Anatomy and Histology, Faculty of Veterinary Science, Bangladesh Agricultural University, Mymensingh, Bangladesh.
2Faculty of Veterinary and Animal Sciences, Gono Bishwabidyalay, Savar, Dhaka, Bangladesh.
3Department of Anatomy and Histology, Faculty of Veterinary and Animal Science, Hajee Mohammad Danesh Science and Technology University, Dinajpur, Bangladesh.
*Corresponding author: Prof. Dr. Ziaul Haque, Department of Anatomy and Histology, Faculty of Veterinary Science, Bangladesh Agricultural University, Mymensingh. Email: zhaqueah80@bau.edu.bd
Received: August 20, 2025 | Accepted: September 10, 2025 | Published: September 25, 2025
Citation: Rupai TE, Ayman U, Akter L, Khan MZI, Awal MA, Parvez MNH, Haque Z, (2025). “Beneficial Effects of Chitosan Oligosaccharides in the Alloxan-Induced Diabetic Mice”. Journal of Veterinary Medicine and Science, 2(2); DOI: 10.61148/3065-7075/JVMS/050.
Copyright: © 2025 Ziaul Haque. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Diabetes mellitus is a long-lasting metabolic disorder that impairs the body's ability to control blood sugar levels. It can give rise to serious complications, including cardiovascular disease, renal dysfunctions, and nerve damage. Type 2 diabetes is often linked to obesity and insulin resistance. Chitosan oligosaccharides (COS) derive from chitosan, have attracted interest for their potential benefits, including antioxidant and anti-inflammatory effects. This study aimed to explore the effects of COS on blood glucose levels, body weight, serum biochemical markers, and pancreatic islets in diabetic mice. We induced diabetes in eight-weeks-old male mice using alloxan @ 110 mg/Kg body weight. The diabetic mice were then divided into five groups: a normal control (NC), a diabetic control (DC), and three treatment groups that received different doses of COS (T1 = 0.5%, T2= 1%, and T3 = 2%) for a period of four weeks. The results showed that COS treatment significantly lowered blood glucose levels in treated groups but the best response was found in T3 group, bringing levels close to normal. The treated mice also showed improvements in body weight gain and serum biochemical markers, including ALT, AST, and creatinine. The pancreatic islets showed a gradual recovery in the treated groups but the most noticeable results were observed in T3 group. These findings suggest that 2% COS might be a promising natural supplement for managing diabetes by lowering blood sugar levels, enhancing overall metabolic health, and protecting the pancreas from further damage.
Chitosan oligosaccharides, diabetes mellitus, blood glucose, pancreatic regeneration, metabolic disorders
1. Introduction:
Diabetes mellitus (DM) is a chronic metabolic disorder of the endocrine system and caused by a confluence of two main factors: improper insulin secretion by pancreatic beta-cells and improper insulin response in insulin-sensitive organs (Galicia-Garcia et al., 2020; Roden & Shulman, 2019). The International Diabetes Federation (IDF) predicted that by 2030, there will be 643 million cases, and by 2045, there will be 783 million cases of diabetes (International Diabetes Federation, IDF Diabetes Atlas-10th Edition). Diabetes patients may not properly produce or use insulin in their bodies and therefore, they have a high blood glucose level and various issues with the metabolism of fats, proteins, and carbohydrates (Schinner et al., 2005). More than 90% of diabetic patients have type 2 DM, according to statistical data and it is mostly caused by obesity and insulin resistance, and increases the risk of cardiovascular diseases. Exercise and anti-diabetic drugs are prioritized in the initial treatment of Type 2 diabetes (DeFronzo & Abdul-Ghani, 2011; DeFronzo et al., 2015; Garvey et al., 2014). A number of fatal consequences, including cardiovascular disease, nephropathy, neurodegeneration, atherosclerosis and retinopathy, may result from persistent failure to maintain normoglycemia (Sarwar et al., 2010; Veiseh et al., 2015). Additionally, hyperglycemia is known to produce reactive oxygen species (ROS), which exacerbate diabetes mellitus and cause both micro and macrovascular problems and these complications result in the patients' deaths (Giacco, 2011; Priyanka et al., 2019; Singhal et al., 2019; Senduran et al., 2020). Controlling blood glucose levels in Type 1 DM and advanced Type 2 DM requires subcutaneous insulin therapy. Tight glycemic control, however, is still a persistent problem for patients with diabetes, and frequent subcutaneous injections may lead to poor patient adherence (Mathieu et al., 2021). As a result, alternative therapies for the treatment of DM have been studied for a long time and numerous bioactive substances produced from plants, animals, and fungi have been demonstrated to have hypoglycemia or anti-diabetic properties (Sarkar et al., 2020; Zhou et al., 2021; Zahnit et al., 2022; Benchikha et al., 2022; Rahman et al., 2022).
Chitin, the second most prevalent natural biopolymer in the world is a polymer that resembles cellulose and can be found in the exoskeletons of crustaceans and molluscan organs, fungi, insects, and yeasts (Tharanathan and Kittur, 2003; Kou et al., 2021). Chitosan (CS), a polysaccharide with cationic structure, is one of the most popular drug carriers and has gained a major attention in diverse biomedical applications, because of its distinctive properties, including low toxicity, biocompatibility, biodegradability, and mucoadhesion (Riva et al., 2011; Shalaby & El-Refaie, 2018; Ahmadifard et al., 2020). Chitosan is a random copolymer of N-acetyl-D-glucosamine and d-glucosamine units that results from the partial degradation of chitin by N-deacetylation in an alkaline environment (Song and Ratner, 2012) connected with β-(1-4) linkage with varying degree of deacetylation.
Chitosan oligosaccharide (COS), which is formed by the depolymerization of chitosan via acid hydrolysis, physical hydrolysis, and enzymatic degradation, is a low-molecular-weight form of chitosan and a nontoxic polyglucosamine (Lodhi et al., 2014; Swiatkiewicz et al., 2015). Chitosan and its oligosaccharide derivatives differ from chitin in that they have antimicrobial (Zheng and Zhu, 2003; Holappa et al., 2006), anti-inflammatory (Yoon et al., 2007; Ma et al., 2011), anti-oxidative (Kim and Thomas, 2007; Yen et al., 2008), and hypocholesterolemic (Liu et al., 2008) properties since they contain reactive, functional groups, such as amino acids and hydroxyl groups.
Chitosan and its derivatives have gained increasing interest recently due to their potential health benefits in the development and management of diabetes, rather than their use as materials in biomedical applications (Katiyar 2011 et al.; Guo et al. 2020). Since prebiotic and biopreservative oligosaccharides are noncariogenic, nondigestible (NDO), and low calorie substances that promote the growth and development of gastrointestinal microflora, there has been an increase in interest in their use in the food industry. In neonatal streptozotocin (STZ)-induced diabetic mice, chitosan is found to have hypoglycemic effects (Miura et al., 1995). According to research by Liu et al. (2007), COS has been shown to increased glucose tolerance and insulin secretion, enhance the impaired pancreatic function in STZ-induced diabetic mice and speed up the proliferation of β-cells in vitro, improving insulin release and glycemic management. COS is typically thought to be biologically more active than chitosan, in addition to sharing some of its properties (Tzeng et al., 2022). COS possesses many functional activities, including the ability to scavenge free radicals, as well as soluble and absorbable qualities whereas in vivo, chitosan has a weak solubility and low absorbability.
However, the efficacy of dietary chitosan oligosaccharides for reducing the incidence of diabetes and cardiovascular diseases- the burning public health issues in Bangladesh yet to be explored. Therefore, the research was designed to examine the efficacy of chitosan oligosaccharides (COS) on the maintenance of health physiology in diabetic mice. The study was conducted to assess the effects of COS on growth rate in diabetic mice, to analyze the efficacy of COS on biochemical profile and to evaluate the role of COS on pancreatic islets in diabetic mice.
2. Materials and Methods
2.1 Selection of Materials
For induction of diabetes, the alloxan was chosen because it preferentially kills pancreatic cells through a variety of mechanisms. Chitosan oligosaccharide (COS), an oligomer of chitosan prepared by the deacetylation of chitin and the second most abundant polymer in nature was selected for this experiment to study the efficacy of dietary chitosan oligosaccharides for reducing the incidence of diabetes and cardiovascular diseases. 2% commercially available powdered chitosan oligosaccharides were used in this regards.
2.2 Evaluation of antidiabetic effects
The chitosan oligosaccharide (COS) was tested for antidiabetic effects. The study was approved by The Animal Welfare and Experimentation Ethics Committee, Bangladesh Agricultural University, Mymensingh, Bangladesh (AWEEC/BAU/19/41) and was carried out according to the current guidelines and developed criteria about study ethics on laboratory animals.
2.2.1. Experimental animals and diets
A total of fifty 8 weeks of old male mice have been collected from ICDDRB, Dhaka. During the 4-week experimental period, all mice have been allowed to access to standard pellets as a basal diets and water ad libitum. Mice were housed in individual cages at 22 ± 3o C and 50 ± 10 % humidity. After 1-week adaptation period, among fifty mice 10 were grouped as- 1) Normal control group (NC), non-diabetic mice; and remaining 40 mice were used to induce diabetes.
2.2.2. Induction, confirmation and validation of diabetes mellitus model
Diabetes was induced by using alloxan @ 110 mg/Kg body weight in mice by single intraperitoneal injection after overnight fasting. Using a Fora Comfort plus mini G71 glucometer made by the ForaCare Switzerland Company, the postprandial glycemia was measured from blood samples (0.5 μl) taken in the morning after 2 h of food intake in overnight fasted mice on the days 1 (24 h after alloxan administration), 2nd, 4th, and 7th. For the following stage of the experiment, mice with blood glucose levels greater than 15 mg/dL were chosen.
2.2.3. Evaluation of antidiabetic efficacy of COS in diabetic mice
The diabetic mice were divided into 4 groups as: 1) diabetic control (DC), without COS supplementation; 2) diabetic mice supplied with 0.5% of COS (T1); 3) diabetic mice supplied with 1% COS (T2); 4) diabetic mice supplied with 2% COS in drinking water (T3). Along with these groups, the normal control (NC) group consisted of 10 healthy mice is also used. To measure the serum glucose level, blood was withdrawn from a tail vein to minimize stress or injury on the days 0, 7, 14, 21 using a Fora Comfort plus mini G71 glucometer made by the ForaCare Switzerland Company.
2.3 Sampling and blood collection
At the end of the experimental period all mice were fasted for 12 hrs, and blood was collected once from orbital sinus of eyes under anesthetic condition to analyze the serum biochemical profile. Before sampling, the body weight of mice was measured using a weighing balance and deeply euthanized within a vacuum glass jar using chloroform.
2.4 Serum preparation and biochemistry
For serum biochemistry, at the next day of blood collection, test tubes containing blood samples were removed from the refrigerator and placed in slanting condition at room temperature for 30 minutes. Blood samples were centrifuged at 3,000×g (Centrifuge 5415C; Eppendorf, Hamburg, Germany) for 15 min to extract serum, and all serum samples were preserved at −80 o C in a deep freezer.
2.5 Collection of pancreas for histopathology
At necropsy, after ventral thoraco-abdominal dissection, pancreas was collected. All visceral organs were examined very carefully for gross changes and recorded carefully. Immediate after gross examination, a piece (1 cubic cm, or suitable size) of collected pancreas was transferred into 10% formalin to fix for histopathology. Plastic cassettes with 10% formalin were used for fixation of samples, overnight. Then the samples went through dehydration and clearing steps and infiltrated in paraffin wax. After trimming, the paraffin blocks were sectioned at 4-6 μm thickness using a rotary microtome (Leica RM 2155, Japan) and collected on glass slides. At next step, the slides were stained with routine stain (haematoxylin and eosin), mounted and finally examined under light microscopes.
2.6 Photography and illustration
Necessary photo of animal rearing during study period and sampling (gross morphometry) were taken using the digital camera (Canon®). The histological pictures of different organs were captured using the digital camera (Canon®). The necessary illustration and contrast adjustment was done by using adobe Photoshop 2019 software.
2.7. Statistical analysis
Shapiro-Wilk test was carried out to evaluate the normality of the data set. During the course of the experiment, data were collected and subjected to statistical analysis using one way of ANOVA approach with post hoc Tukey’s multiple comparisons test with a completely randomized design (CRD) using Graph Pad Prism (version 9.0). Significant means were separated using multiple comparisons at 5% level of significance. All data points (n-numbers) are plotted in each bar graph (three independent experiments).
3. Results
3.1. Effect of COS on body weight
The mean body weight of mice of various groups on different day of the experiment is shown in Figure 1. The mean body weight was increased with the growing age of the mice in the normal control group (NC) and decreased in the diabetic control group (DC), whereas the COS treated groups (T1, T2, T3) showed a gradual increase in body weight with the increased concentration of COS which is statistically significant.
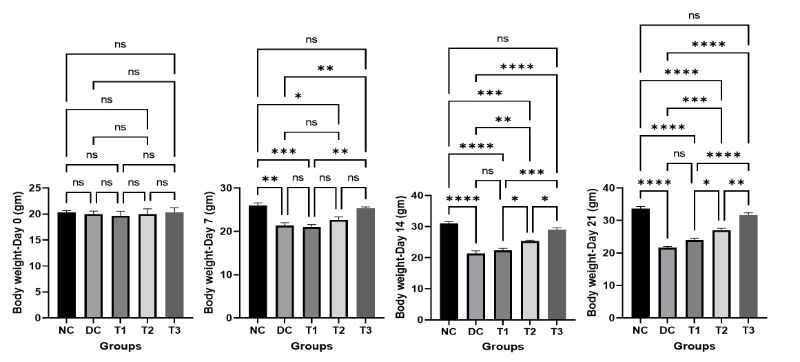
Figure 1: Effects of COS on body weight of mice at different concentration and different time periods (Mean ± SEM). DC = control, T1 = 0.5% COS, T2 = 1% COS, T3 = 2% COS with basal diet. Significance was considered at the level of 5% (P < 0.05). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.005, ****P ≤ 0.001, ns = non-significant.
There were non-significant differences among the control and the highest dosage treatment group (T3) on different days of experiment that reveals the propitious improvement of the final body weight of mice in group T3.
3.2. Effects of COS on blood glucose concentration
The concentration of blood glucose of mice of various groups on different day of the experiment is shown in Figure 2. The result showed that the blood glucose concentration increased very little with the growing age of the mice in normal control group (NC) which is physiologically congruent, but increased significantly in diabetic control group (DC) compared to the normal control group. The COS treated groups (T1, T2, T3) showed a gradual decrease in blood glucose concentration with the increased concentration of COS which is statistically significant compared to diabetic control group (DC). The significant difference was also present among COS treated groups and the lowest value of blood glucose concentration was found in group T3 at day 21 which was not significant with normal control group which indicates the positive effects of high dose of COS.
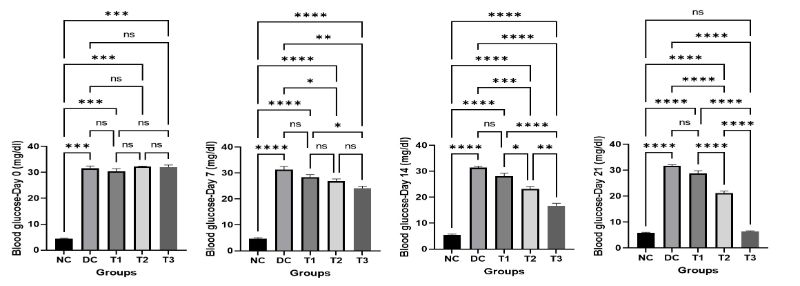
Figure 2: Effects of COS on blood glucose of mice at different concentration and different time periods (Mean ± SEM). DC = control, T1 = 0.5% COS, T2 = 1% COS, T3 = 2% COS with basal diet. Significance was considered at the level of 5% (P < 0.05). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.005, ****P ≤ 0.001, ns = non-significant.
3. Effects of COS on ALT and AST
The results of serum ALT and AST of normal control (NC), diabetic control (DC), COS treated groups (T1, T2, T3) of mice on different day of the experiment are revealed in Figure 3 and Figure4. At Day 0, the result showed that the serum ALT and AST were significantly higher in diabetic control group (DC) and COS treated groups (T1, T2, T3) compared to the normal control group (NC). But with advancement of the experiment it was found that a gradual decrease in the serum ALT and AST was evident in COS treated groups (T1, T2, T3) at day 7, 14, 21 which is statistically significant compared to diabetic control group (DC). The values of serum ALT and AST were increased in diabetic control group (DC) with the passage of time but the treatment with COS showed lowering of these values and most propitious and auspicious result was found in group T3 at day 21 which is almost near to normal control group (NC).
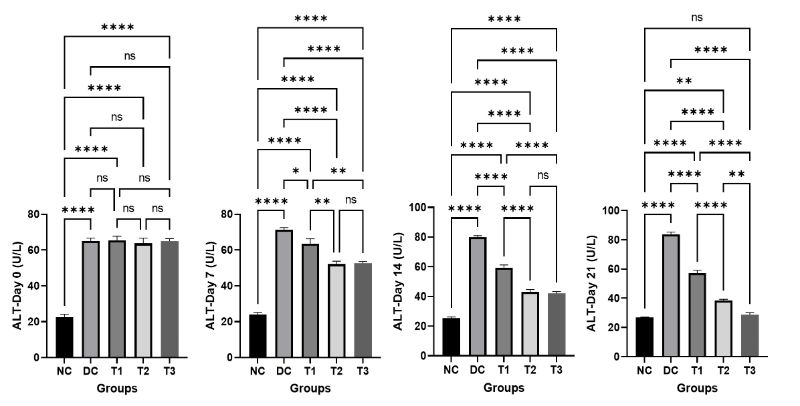
Figure 3: Effects of COS on serum ALT of mice at different concentration and different time periods (Mean ± SEM). DC = control, T1 = 0.5% COS, T2 = 1% COS, T3 = 2% COS with basal diet. Significance was considered at the level of 5% (P < 0.05). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.005, ****P ≤ 0.001, ns = non-significant.
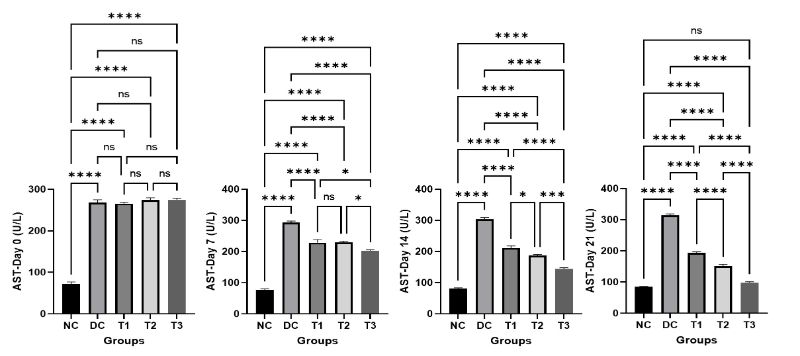
Figure 4: Effects of COS on serum AST of mice at different concentration and different time periods (Mean ± SEM). DC = control, T1 = 0.5% COS, T2 = 1% COS, T3 = 2% COS with basal diet. Significance was considered at the level of 5% (P < 0.05). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.005, ****P ≤ 0.001, ns = non-significant.
4. Effects of COS on serum creatinine
The results of serum creatinine of normal control (NC), diabetic control (DC), COS treated groups (T1, T2, T3) of mice on different day of the experiment are revealed in Figure 5. At the beginning of the study, the value of creatinine in normal control group is significantly lower compared to diabetic control group (DC) and COS treated groups (T1, T2, T3). With the passage of time the level of creatinine in diabetic control group (DC) was increased gradually but in COS treated groups (T1, T2, T3) a gradual decrease was found at day 7, 14, 21. The treatment with COS showed returning of creatinine level in normal range and most excellent result was found in group T3 at day 21. There is no significant difference between normal control (NC) and T3 group.
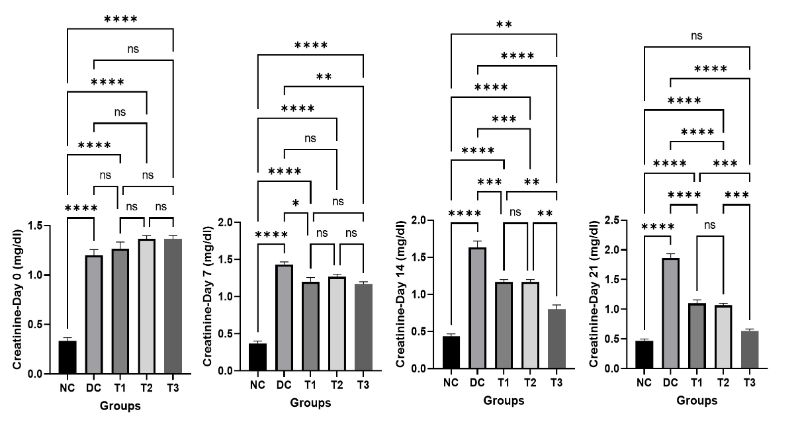
Figure 5: Effects of COS on creatinine of mice at different concentration and different time periods (Mean ± SEM). DC = control, T1 = 0.5% COS, T2 = 1% COS, T3 = 2% COS with basal diet. Significance was considered at the level of 5% (P < 0.05). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.005, ****P ≤ 0.001, ns = non-significant.
5. Effects of COS on pancreatic islets
In normal control group (NC), the pancreatic acini and pancreatic islets showed normal histological architecture (Figure 6, NC). In diabetic control group, the pancreatic islets showed a great loss and degeneration of cells (Figure. 6, DC). After the ending of the experiment, the COS treated groups showed a variety level of recovery of the pancreatic islets which were affected by diabetes with increasing concentration of COS (Figure. 6, T1, T2, T3). In group T1 (0.5% COS), the cells of pancreatic islets did not show much improvement and the cells were degenerated and necrosed, but regeneration of cells was clearly visible in group T2 (1% COS). The regeneration of cells of pancreatic islets in group T3 (2% COS) was at the greater extend and the histoarchitecture was almost near to the normal control group (NC) which indicates the recovery of diabetic pancreas with COS treatment at a higher concentration.
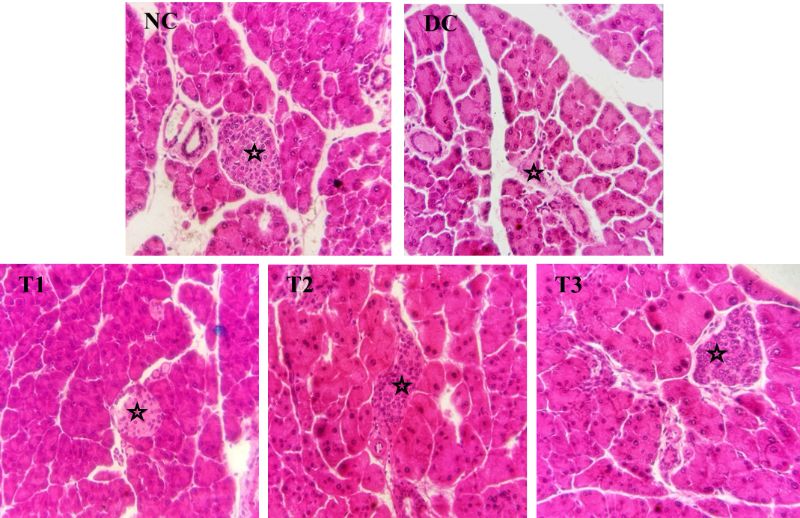
Figure 6: Effects of COS on pancreatic islets of mice of different groups. Asterisks indicate pancreatic islets (20X). NC = Normal control, DC = Diabetic control, T1 = 0.5% COS, T2 = 1% COS, T3 = 2% COS.
4. Discussion
Diabetes mellitus, the most prevalent disease in the world is characterized by a substantial elevation of blood glucose levels, is a more complex metabolic syndrome. It has been shown that chitosan plays a favourable role in the maintenance and growth of pancreatic cells, in lowering blood sugar levels, and in preventing diabetes mellitus linked to poor lipid metabolism (Othman et al., 2021). Contrary to high molecular weight chitosan, COS can quickly enter the bloodstream and have systemic biological effects on the body because of their shorter chains, free amino groups in D-glucosamine units, and ease of absorption through the GI tract.
In the present study, the mean body weight of the diabetic mice was significantly decreased with the advancement of the study. Whereas treatment with the COS showed a gradual increase in body weight with the increased concentration of COS. This result is in line with the findings of some earlier researchers in diabetic mice treated with COS (Katiyar et al., 2011; Kim et al., 2014). Dietary COS supplementation has been shown to enhance growth performance significantly like body weight, fed conversion ratio, nutrient digestability etc. in broilers (Huang et al., 2005; Shi et al., 2005; Li et al., 2007) and weaned pigs (Liu et al, 2008b; Zhou et al., 2012). Improved small intestinal morphological structure and higher growth hormone and insulin-like growth factor concentrations in serum may be the mechanisms underlying dietary COS's ability to boost growth performance (Tang et al., 2005; Chen et al., 2009; Xu et al., 2014) which is also found in the present study.
The postprandial glycemia was measured from blood samples of diabetic mice in the present study using glucometer. The result showed that the blood glucose concentration increased significantly in diabetic mice induced by alloxan monohydrate. Alloxan was reported to cause a massive reduction in insulin release by the destruction of β-cells of the islets of Langerhans and thereby induce hyperglycemia (Siyem et al., 2002). The COS treated groups showed a significant decrease in blood glucose concentration with the increased concentration of COS. The lowest value of blood glucose concentration was found in group highest dose of COS (2%) at day 21 which was near to the non-diabetic mice. The results of the current investigation clearly indicate that the COS has hypoglycemic effect on alloxan induced diabetic mice at higher concentration. Similarly, hypoglycemic effect of COS has also been described by some authors earlier in diabetic mice and rats (Katiyar et al., 2011; Kim et al., 2014; Zheng et al., 2018).
COS has a positive impact on serum biochemical profile described by many authors. The most common indicators for assessing liver function are trans-aminases (alanine aminotransferase - ALT, aspartate aminotransferase - AST), lactate-dehydrogenase (LDH), and bilirubin. In the present study, treatment with COS effectively reduced the serum AST and ALT with the increasing concentration of COS in diabetic mice and most efficient result was found in 2% COS supplemented group. COS displays protective effects in coronary heart disease via enhanced antioxidant capacity and decreased serum AST and ALT level (Jiang et al., 2019). Serum AST and ALT activities were effectively decreased in carbon tetrachloride-induced liver damage in mice when treated with COS (Yan et al., 2006).
Diabetic nephropathy, a common side effect of diabetes mellitus that can result in renal failure. Utilizing urea, creatinine, and uric acid markers, the renal function of diabetic animals are usually assessed. When the kidneys are damaged, there will be an accumulation of creatinine in the blood, which can be seen in diabetes as a complication of it (Cao et al., 2016). Increased creatinine level was seen in diabetic mice in the current study which is further reduced significantly in COS treated groups especially in 2% COS group as supportive of nephroprotective effects. It is reported in diabetic rats that COS supplementation significantly lowered plasma glucose, creatinine, uric acid, triglyceride (TG), and total cholesterol (TC) levels (Liu et al., 2021). Low molecular weight chitosan oligosaccharide (GO2KA1) supplements in diabetic mice showed a significant reduction in total cholesterol (Kim et al., 2014). Thus COS may provide propitious and favourable influence on the serum biochemical profile of diabetic animals.
β-cells are responsible for insulin production, which is synthesized as pre-proinsulin (Bunney et al., 2017). Traditional research has linked β-cell malfunction to β-cell death and there is a possibility that the malfunctioning of β-cells in Type 2 DM may result from a more intricate network of interactions between the environment and several biochemical processes involved in cell biology (Christensen & Gannon, 2019; Halban et al., 2014). An investigation of the pancreas' morphology in the diabetic rat group revealed varying degrees of islet loss, β-cell loss, and nuclear pyknosis of cells (Yuan et al., 2009). In the present study, the histopathology of pancreatic islets showed a great loss and degeneration of cells in alloxan induced diabetic mice. The COS treatment showed a varying level of reverse condition that means the lost pancreatic islet cells started increasing proliferation in number. And the histoarchitecture of pancreatic islets in 2% COS mice was very close to the normal control mice which offers great promise for the treatment of diabetes mellitus with COS treatment at a higher concentration. According to Kim et al. (2014) the increased pancreatic weight in diabetic mice following COS therapy served as an indirect demonstration of increased insulin production by proliferated β-cells. With an increase in COS concentration, the severity of pancreatic islets cell injury reduced in diabetic rats (Yuan et al., 2009). They reported that COS may enhance antioxidant capacity and protect pancreatic islets from STZ-induced diabetes in rats. COS effectively sped up the growth of pancreatic islet cells and had a direct impact on pancreatic cells and insulin production in diabetic rats described by Liu et al. (2007).
A drive is currently underway for nutritional supplements derived from natural sources, which may be a better option than the anti-diabetic drugs that are already available over the counter. COS has positive effects on body weight, hypoglycemic effect, lowering of serum AST, ALT and creatinine; increase proliferation of islet-cells in the pancreas. Based on the findings of the present study and above discussion it is clear that chitosan with excellent biological properties, can be a potent anti-diabetic supplement.
5. Conclusions
The findings of this study confirm the positive effects of chitosan oligosaccharides (COS) in diabetic mice induced by alloxan. COS supplementation helped restore body weight, significantly reduced blood glucose, and improved important serum biochemical markers like ALT, AST, and creatinine. Histopathological analysis showed significant regeneration of pancreatic islets, especially in the 2% COS group, which looked very similar to the normal control. Overall, COS, particularly at higher doses, seems to be a useful natural supplement for managing diabetes, regulating metabolism, and protecting the pancreas.