International Surgery Case Reports
OPEN ACCESS | Volume 7 - Issue 3 - 2025
ISSN No: 2836-2845 | Journal DOI: 10.61148/2836-2845/ISCR
Guilherme Centofante1, Bruna De Fina2, Henrique Elkis3, Rafael Noronha Cavalcante4, Matheus Rocha Araujo5, Francisco Leonardo Galastri 6, Ricardo Virginio dos Santos7, Rafael de Athayde Soares2*
1,2Hospital Paulistano, Hospital Samaritano Paulista, Hospital Metropolitano Lapa, Hospital Nove de Julho.
3,4,5Hospital Paulistano, Hospital Samaritano Paulista, Hospital Metropolitano Lapa.
6,7,8 Hospital Nove de Julho.
*Corresponding author: Rafael de Athayde Soares, Hospital do Servidor Público Estadual de São Paulo, IAMSPE.
Received: July 10, 2025 |Accepted: July 31, 2025 |Published: August 04, 2025
Citation: Guilherme Centofante, Rafael de Athayde Soares. (2025) “The experience of Mechanical Thrombectomy of Pulmonary Emboli With Use of the Lightning FlashTM: a Case Series Report.”, International Surgery Case Reports, 7(2); DOI: 10.61148/2836-2845/ISCR/103.
Copyright: © 2025. Rafael de Athayde Soares. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background: The burden of Pulmonary embolism (PE) is explained by the fact that it is the third leading cause of cardiovascular death. Over the last 2 decades, the steady rise in incidence and stagnant 10% overall 30-day mortality rate underscore the unmet need for more effective PE treatment with advanced therapy.
Methods: This case series report about patients with PE submitted to Computer-Assisted Vacuum Thrombectomy (CAVTä) with the Indigoä System Lightning FlashTM. The main objective of this study is to describe a case series report on the experience of using Lightning FlashTM aspiration system (Penumbra, Inc.) for PE.
Results: there were nine patients submitted to mechanical thrombectomy (MT) with Lightning FlashTM (Penumbra, Inc.) due to PE. The mean age was 55,89 years and most of the patients had a high-intermediate risk for PE (88.9%). The mean right ventricle (RV)–to–left ventricle (LV) ratio before thrombectomy was 1,11, reducing to 0,75 48hs after MT (p = 0.042). The mean blood loss was 266,67ml, and no patients required blood transfusion during the procedures. The mean time of MT was 18.33 min, the mean time of procedure was 49.44 min, and the mean pre-procedure pulmonary artery pressure (mPAP) was 60,11mmHg, decreasing to 47,75mmHg after the procedure (p = 0.039). There were no major adverse events or deaths related to the procedures. Technical success was 100% and the perioperative mortality rate was 0%.
Conclusion: This study showed significant improvement in right ventricular function, no cases of perioperative mortality, no events of major bleeding, no cardiac injury, and fast procedure times. Thrombectomy with the use of Indigo System Lightning FlashTM (Penumbra, Inc.) demonstrated safety and efficacy endpoints for the treatment of acute PE and may be considered by endovascular physicians for use in intermediate-risk PE. Larger, randomized studies should be performed to properly evaluate the outcomes and device-related serious adverse events.
The burden of Pulmonary embolism (PE) is explained by the fact that it is the third leading cause of cardiovascular death. Over the last 2 decades, the steady rise in incidence and stagnant 10% overall 30-day mortality rate underscore the unmet need for more effective PE treatment with advanced therapy.1,2 Currently treatments for pulmonary embolism are divided into 4 categories: systemic anticoagulation, systemic thrombolysis (ST), catheter-directed thrombolysis (CDT), and mechanical thrombectomy (MT).
There is consensus that high-risk PE, which is defined by hemodynamic instability, necessitates rapid reperfusion treatment due to an early mortality rate exceeding 30%.3 In general, MT has several advantages over CDT and ST in the treatment of submassive and potentially massive PE, due to the shorter length of time to restore the blood flow and reduced risk of bleeding.
Recently, some published trials compared the different forms of treatment for PE. In the SEATTLE II (Submassive and Massive Pulmonary Embolism Treatment With Ultrasound Accelerated Thrombolysis Therapy) trial, which evaluated CDT, thrombolytic agents were associated with a >10% rate of bleeding complications.4 The results in an ST trial, PEITHO (Pulmonary Embolism Thrombolysis Study), were similar.5 In the SEATTLE II trial, thrombolytic agents were given to patients in intensive care for 12 to 24 hours; in the more recent OPTALYSE PE (Optimum Duration of Acoustic Pulse Thrombolysis Procedure in Acute Pulmonary Embolism) trial, CDT was performed in as short a time as 4 hours.6
The EXTRACT-PE (Evaluating the Safety and Efficacy of the Indigo Aspiration System in Acute Pulmonary Embolism) trial evaluated 119 patients with submassive PE and showed significantly reduced right ventricular-to-left ventricular ratios at 48 hours (0.43 ratio reduction; 95% CI, 0.38–0.47; P <0.0001). In addition, procedural pulmonary artery (PA) pressures were reduced in as little as 37 minutes of median overall procedure time. Adjunctive thrombolytic use was minimal (1.7% of patients) and safe (rates of pulmonary vascular injury, clinical deterioration, and major bleeding at 48 hr, all 1.7%; 30-d mortality rate, 2.5%).7
Recently, a new device has been introduced to perform MT in patients with submassive/massive PE. Indigo System Lightning FlashTM (Penumbra, Inc) is a next-generation catheter-directed aspiration thrombectomy system, which has recently been introduced to the European market for the reperfusion treatment of acute PE. Larger size, 16 French aspiration catheter, and the innovative computer-assisted vacuum thrombectomy system potentially translate into improved efficacy and safety.8
The main objective of this study is to describe a case series report experience about the use of Lightning FlashTM Intelligent Aspiration systems (Penumbra, Inc.) for PE.
Methods
This is a case series report about patients with PE submitted to Computer-Assisted Vacuum Thrombectomy (CAVTä) with the Indigo System Lightning FlashTM. The primary effectiveness endpoint is the change in the right ventricle (RV)–to-left ventricle (LV) ratio from before the thrombectomy to 48 hours after the procedure. RV/LV ratio is available at both imaging times and acquired by using the same imaging modality (computed tomography pulmonary angiography or echocardiography).
The primary safety endpoint is a composite of major adverse events (MAEs)—device-related death, major bleeding, device-related clinical deterioration, device-related pulmonary vascular injury, and device-related cardiac injury—48 hours after the procedure.
The secondary outcomes are the incidences of device-related serious adverse events (SAEs), all-cause mortality within 30 days, and symptomatic PE recurrence within 30 days.
All statistical analyses were performed by using SPSS 21.0 for Mac. Descriptive statistics were calculated. Median results were calculated by comparative medians tests, using ANOVA and Wilcoxon tests. P < 0,05 was defined as significant.
Technique Description
Lightning Flash features dual clot detection algorithms, improving upon the singular algorithm featured in Lightning 12 and 7. One algorithm detects clot based on pressure differentiation, while the other algorithm detects the interaction of flow through the system. The communication between these two algorithms results in rapid recognition of whether the catheter is actively engaging clot or is in patent flow, which is designed to maximize case efficiency. 9
The Lightning Flash catheter is 16-F sheath compatible. The catheter features MaxID technology, which allows for a large inner diameter comparable to large-bore catheters while still maintaining a lower profile. The Lightning Flash catheter was engineered to optimize its lower profile size to make it extremely trackable and atraumatic but also very powerful, giving the capability to navigate through tortuous anatomy and remove heavy thrombus burden. Despite the increase in size, the Lightning Flash catheter is two times softer than Lightning 12. This catheter is available in HTORQ and XTORQ tip shapes, designed for optimal engagement of wall-adherent thrombus regardless of the size of the vessel. Lightning Flash comes packaged with a 6-F Select+ Access Catheter. The Select+ Catheter is designed for excellent tracking and navigation, allowing for further deliverability and vessel selection. Penumbra’s Lightning System is the only computer-assisted vacuum thrombectomy device on the United States market. The combination of dual clot detection algorithms and MaxID technology enable Lightning Flash to be minimally invasive and maximally effective, thus resulting in the most powerful and advanced thrombectomy device for pulmonary embolism (PE) and venous thrombus. This allows for the potential to remove a higher volume of thrombus in less device time.10
Procedural Details
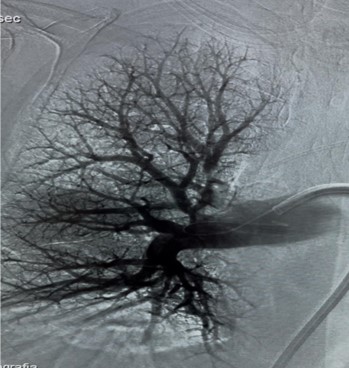
Usually, access was gained in the right femoral vein, and a 16-F, 65-cm Gore DrySeal sheath (Gore & Associates) was introduced into the vasculature and placed in the right or left main pulmonary artery (PA). A Lightning Flash HTORQ was then advanced into the right main PA. To place Lightning Flash into the correct anatomy, the catheter was deftly tracked over a 6-F Select Catheter (Penumbra, Inc.) with a Bernstein tip shape and a 0.035-inch Amplatz guidewire (Boston Scientific Corporation). With the catheter in place, aspiration was initiated. In 2.5 minutes of device time, the thrombus burden was extracted from the right or left main PA (Figure 1), and pulmonary angiography displayed considerable improvement from the beginning of the case (Figure 2).
Figure 1: Thrombus burden was extracted from the left main pulmonary artery
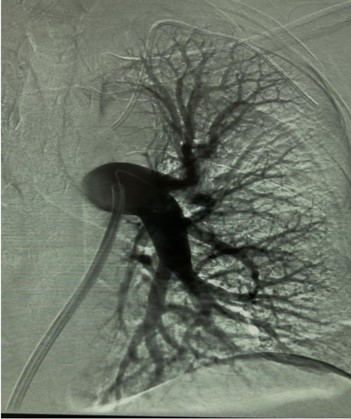
Figure 2: Right Pulmonary artery post-operative image.
Results:
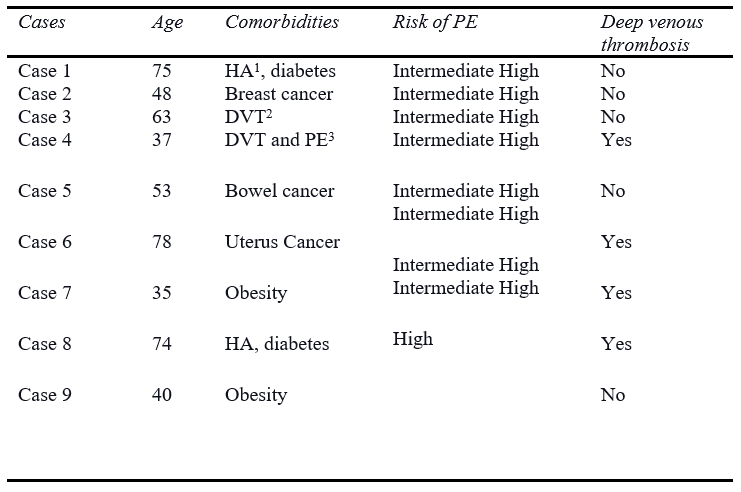
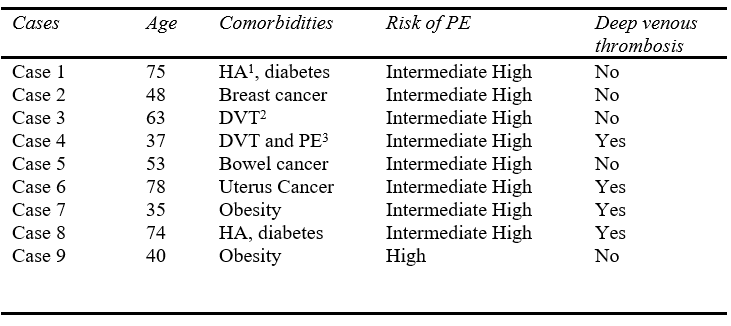
Regarding the results, there were nine patients submitted to MT with Lightning Flash (Penumbra, Inc.) due to PE. The mean age was 55,89 years and most of the patients had a high intermediate risk for PE (88.9%). Concerning the comorbidities, 2 patients had arterial hypertension and diabetes, 2 patients previous history of deep venous thrombosis, 3 patients had active cancer, and 2 patients had severe obesity. Furthermore, four patients were diagnosed with acute deep venous thrombosis in lower limbs during the PE. (Details in Table I).
Table I: Clinical Data of the patients
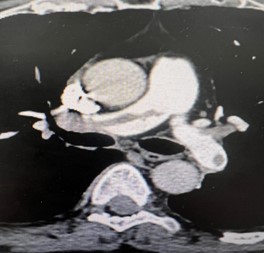
All patients were submitted to a preoperative pulmonary CT-SCAN, showing a large thrombus burden at main pulmonary arteries (figures 3 and 4).
Figure 3: CT-Scan demonstrating large thrombus burden at left pulmonary artery
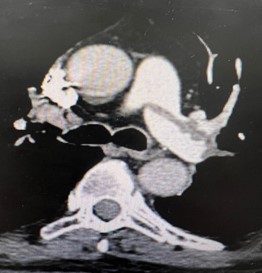
Figure 4: CT-Scan demonstrating large thrombus burden at righ and left pulmonary artery
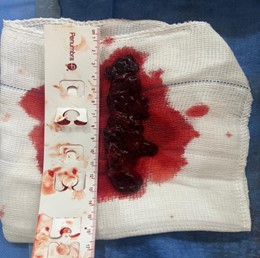
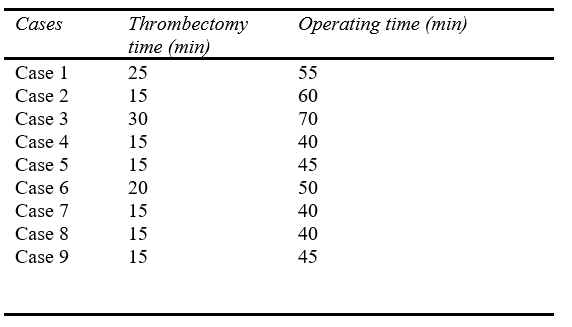
Moreover, concerning the technical data of the procedures, the mean right ventricle (RV)–to–left ventricle (LV) ratio before thrombectomy was 1,11, reducing to 0,75 48hs after MT (p = 0.042). The mean blood loss was 266.67ml, and no patients required blood transfusion during the procedures. The mean time of MT was 18.33 min, the mean time of operating was 49.44 min, and the mean pre-procedure pulmonary artery pressure was 60,11mmHg, decreasing to 47,75mmHg after the procedure (p = 0.039) (figure 5).
Figure 5: Thrombi removal after MT
The mean tricuspid annular plane systolic excursion (TAPSE) preoperative was 14.24mmHg preoperative, ranging to 17.88mmHg post-operative (p = 0.042). Those data demonstrate an increase in the hemodynamic status of the patients after the MT. There were no major events or deaths related to the procedures. Technical success was 100% and the perioperative mortality rate was 0% (Details in Tables II and III).
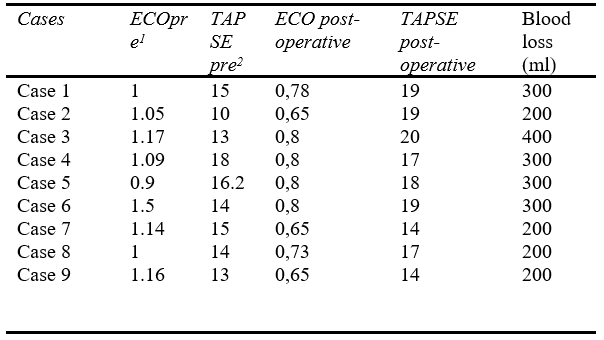
1 – arterial hypertension
2 – previous deep venous thrombosis
3 – previous pulmonary embolism
Table II: Technical procedures data
1 - Right ventricle (RV)–to–left ventricle (LV) ratio
2 - The meaning tricuspid annular plane systolic excursion
Table III: Technical procedures data
Discussion
This paper presents a challenging case series report of patients with PE submitted to MT with Lightning Flash. Recently, the STRIKE-PE study was the first report on the use of computer-assisted vacuum thrombectomy (CAVT) with the Indigo Aspiration System (Penumbra, Alameda, California).11 The authors reported 150 consecutive patients treated with CAVT, with a mean age of 61,3 years, 54.7% men and 94.7% presenting intermediate-risk PE. Moreover, Median thrombectomy and procedure times were 33.5 minutes and 70.0 minutes, respectively, resulting in a mean reduction in systolic pulmonary artery pressure of 16.3% (P < .001). The mean RV/LV ratio decreased from 1.39 to 1.01 at 48 hours, a 25.7% reduction (P < .001). Four (2.7%) patients experienced a composite MAE within 48 hours. The authors concluded that Interim results from the STRIKE-PE study demonstrated a significant reduction in pulmonary artery pressure and RV/LV ratio, promoting significant improvements in 90-day functional and QoL outcomes in patients with PE. These data are comparable with those found in this present case series report, whereas the mean right ventricle (RV)–to–left ventricle (LV) ratio before thrombectomy was 1.11, reducing to 0.75 48hs after MT (p = 0.042) demonstrating an increasing of the hemodynamic status of the patients after the MT. Moreover, the mean thrombectomy and procedure times were 18.33min and 49.44min respectively, demonstrating the feasibility and safety of the MT procedures.
Acute PE encompasses a heterogeneous spectrum of disease manifestation and severity with considerable mortality rates and unsatisfactory long-term outcomes of advanced stages. Hemodynamic deterioration in PE results from right ventricular pressure overload, leading to progressive RV failure and subsequently to the development of the spiral of cardiogenic shock and death. The estimated early mortality is up to 30% in high-risk (HR) PE and up to 15% in intermediate-risk (IHR) PE.12 Submassive PE, defined by normal blood pressure but right ventricular (RV) dysfunction, carries the best therapeutic approach uncertainty. Although anticoagulation alone does not prevent death or clinical deterioration in 5% of these patients, systemic thrombolysis, the most extensively studied active thrombus treatment strategy, carries a high risk of major bleeding.13 In a meta-analysis evaluating 866 submassive PE patients treated with thrombolytics and 889 treated with anticoagulation alone, all-cause mortality was lower in the thrombolytic group than in the anticoagulation group (1.39% vs. 2.92%). However, rates of major bleeding were significantly higher in the thrombolytic group (7.74% vs. 2.25%).14
To avoid the high-risk bleeding rates of systemic thrombolysis, catheter-directed thrombolysis, and percutaneous mechanical aspiration have been developed to perform a local activity in thrombolysis at pulmonary circulation. The SEATTLE II (A Prospective, Single-Arm, Multicenter Trial of Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism) study enrolled 150 acute PE patients treated with ultrasound-assisted catheter-directed thrombolysis and reported an associated reduction in the RV-to-left ventricular (RV/LV) ratio at 48 h, but also a 10% major bleeding rate within 72 h of treatment.7 In a recent meta-analysis of 860 patients treated with catheter-directed thrombolysis and ultrasound-assisted catheter-directed thrombolysis, it was observed that the patients had an average RV/LV ratio reduction of 0.34.15 In the FLARE (A Prospective, Single-Arm, Multicenter Trial of Catheter-Directed Mechanical Thrombectomy for Intermediate-Risk Acute Pulmonary Embolism) study which prospectively evaluated the FlowTriever System (Inari Medical, Irvine, California), patients treated with the device had an average RV/LV ratio reduction of 0.38.16
In this present case report study, the major bleeding was 0%. No patients needed a blood transfusion. The major bleeding rate in the EXTRACT-PE study was 1.7%.7 Overall, the bleeding rate is lower in MT than in thrombolysis trials: major bleeding occurred in 11.5% of patients in the systemic thrombolysis trial, PEITHO (Pulmonary Embolism Thrombolysis Trial5 and 10% of patients in the SEATTLE II ultrasound-assisted catheter-directed thrombolysis study.7 The low bleeding rate is encouraging and reflects the fact that thrombus aspiration does not require a thrombolytic agent, demonstrating the feasibility and safety of the MT procedures.
Another important data is that the patients in this present paper had no cardiac injuries during 16F aspiration catheter passage through the right heart. Indeed, the STRIKE-PE study evaluating 150 patients showed that one patient experienced major bleeding, device-related pulmonary vascular injury, and device-related clinical deterioration due to a pulmonary artery perforation (device-related serious adverse event - SAE; the patient fully recovered. Another patient experienced major bleeding and device-related clinical deterioration due to pulmonary artery hemorrhage (device-related SAE).11 Previously, the EXTRACT-PE study showed that no cardiac injury occurred, indicating that the 8-F Indigo aspiration catheter could pass through the right heart with minimal risk for cardiac injury. However, 2 events qualified as pulmonary vascular injury; one was related to a small perforation likely from the guidewire, and the other was due to a distal vessel perforation after multiple passes were attempted. The Indigo Separator is advanced and retracted out of and back into the Indigo aspiration catheter to facilitate the clearing of the thrombus from the Indigo aspiration catheter tip. Although the aspiration catheter has a soft atraumatic edge, excessive manipulation of a guidewire or the separator in distal anatomy can lead to vascular injury.7
This study has some limitations since it is a case-series report with too small sample size, lack of randomization, a comparator arm, and no longer follow-up. Larger, randomized studies should be performed to properly evaluate the outcomes and device-related serious adverse events.
Conclusion
This study showed significant improvement in right ventricular function, no cases of perioperative mortality, no events of major bleeding, no cardiac injury, and fast procedure times. Thrombectomy with the use of Lightning Flash (Penumbra, Inc.) demonstrated safety and efficacy endpoints for the treatment of acute PE and may be considered by endovascular physicians for use in intermediate-risk PE. Larger, randomized studies should be performed to properly evaluate the outcomes and device-related serious adverse events.
The Authors declare that there is no conflict of interest regarding this paper.
Informed consent has been obtained from the patient for publication of the case report and accompanying images.