International Journal of Epidemiology And Public Health Research
OPEN ACCESS | Volume 9 - Issue 1 - 2026
ISSN No: 2836-2810 | Journal DOI: 10.61148/2836-2810/IJEPHR
Eduardo Souza Dinato1, Alcione da Silva Fernandes Dinato1, Geovanna Eduarda Dias Cardoso1, Stefany Silva Dias2, Izabelly Vitória Pacheco Davi2, Mariana Fabian Oliveira Dias2, Thaynara Rodrigues Borges2, Sérgio Eustáquio Lemos da Silva1,2*
1Veterinary Medicine Course, Triângulo University Center, UNITRI, Brazil.
2Biomedicine Course, Triângulo University Center, UNITRI, Brazil.
*Corresponding author: Sérgio Eustáquio Lemos da Silva, Veterinary Medicine Course, Triângulo University Center, UNITRI, Brazil., Biomedicine Course, Triângulo University Center, UNITRI, Brazil.
Received: May 19, 2025
Accepted: May 24, 2025
Published: May 28, 2025
Citation: Eduardo S Dinato, Fernandes Dinato A D S, Dias Cardoso G E, Stefany S Dias, Pacheco Davi I V, Oliveira Dias M F, Thaynara R Borges, Lemos da Silva S E. (2025) “Immunodiagnosis as Tool for Determining Epidemiological Indicators of Bovine Viral Diarrhea and Associated Risk Factors”. International Journal of Epidemiology and Public Health Research, 6(4); DOI: 10.61148/2836-2810/IJEPHR/136.
Copyright: © 2025. Sérgio Eustáquio Lemos da Silva. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited., provided the original work is properly cited.
Bovine Viral Diarrhea is an infectious disease of reproductive nature, transmitted by direct and indirect contact, characterized by causing diarrhea and erosion of the intestinal mucosa, causing several economic problems to producers in Brazil and worldwide. It is caused by a Pestivirus, with great genomic and antigenic diversity, having two biotypes, one cytopathogenic and the other non-cytopathogenic, which results in transient and persistent forms of infection, and can also manifest subclinically or fatally in more severe cases. BVDV has two important genotypes, BVDV 1 and BVDV 2, resulting from mutations and rearrangements that occur in the E2 glycoprotein. The main means of contamination of BVD are PI animals.
The objective of this study was to analyze epidemiological indicators through immunodiagnosis and risk factors that predispose to new cases. Between 1987 and 2002, 6,512 animals were analyzed by sn and ELISA test methods, of which 2,408 were reactive for BVD, showing a relative prevalence and morbidity of 37%. In the years between 2003 and 2021, 28,929 animals were examined, from 3,168 properties in different regions of the country, where 16,024 animals presented antibodies for infection, the seroprevalence detected was 55.39%. The morbidity found within the properties in the herds was 78.75%. The serological surveys carried out during this period showed a high prevalence rate of the disease, such as in the South 75.68%, Central-West 64%, Southeast 50.60%, and Northeast 45.02%. Given the severity of BVD and the range of symptoms, it is necessary to perform a differential diagnosis with other diseases, and it is essential to create prophylactic programs, as well as to raise awareness among producers so that more effective biosecurity measures can be used to reduce the damage caused and viral spread.
1. Introduction
Brazil is the largest producer and exporter of beef in the world, with an estimated herd of approximately 238.6 million cattle in 2023, according to the Brazilian Institute of Geography and Statistics (IBGE) [26]. Dairy farming has also been gaining prominence and demonstrating continuous growth in herd population and production, placing Brazil as the sixth largest milk producer globally. In view of this, Brazilian cattle farming, whether for dairy or beef production, faces several challenges in its routine, due to infectious diseases, among which the infections of greatest concern to producers are reproductive diseases, among which Bovine Viral Diarrhea (BVD) stands out [3, 11, 49].
The first report on BVD occurred in 1964 by Olafson et al. It is an infectious disease of great pathogenicity and complexity, has a cosmopolitan distribution and is widely disseminated in Brazilian herds, and is considered one of the most important pathogens for cattle farming, and causes enormous impacts and financial losses in livestock farming in Brazil and the world, and can be spread through direct and indirect contact, has a high morbidity rate, can affect the entire herd, and is characterized by causing diarrhea and erosions in one of the most important pathogens for cattle farming, and causes enormous impacts and financial losses in livestock farming in Brazil and the world, and can be spread through direct and indirect contact, has a high morbidity rate, can affect the entire herd, and is characterized by causing diarrhea and erosions in the gastrointestinal mucosa due to its virulence, and can cause the death of animals in more severe cases [17, 48, 51].
In Brazil, the first cases of infection occurred in the late 1960s, initially associated with gastroenteric disease, later correlated with a wide variety of clinical and subclinical symptoms, such as respiratory, digestive, reproductive, hemorrhagic, cutaneous and immunosuppression problems, and may present from latent contamination without presenting symptoms or with few clinical manifestations, or even be an acute and fatal disease as in the cases of Mucosal Disease [11, 18, 22, 23, 38].
Although it presents a complex of symptoms, BVD is characterized mainly as a reproductive disease, in which its presence in herds can cause several problems and reproductive failures, leading to great losses directly and indirectly in the livestock production of these animals, without initially raising any suspicion on the part of the owners and veterinarians responsible. Another worrying factor is the fact of fetal contamination and the birth of calves with persistent infection (PI), since these animals constitute the main source of contagion, being responsible for the spread and maintenance of the virus in herds, in addition to making it difficult to control and prevent contamination [11, 18, 24, 33].
Given the importance of contamination and the economic severity caused to cattle producers, since, in most cases, contagion and the realization of an assertive diagnosis only occur after the installation and propagation of the agent in the herd [32], it was necessary to carry out this present study, with the general and specific objective of analyzing the epidemiological situation and the harm caused by the disease in the country today, also aiming to assist in taking more efficient biosecurity and prophylaxis measures and greater agility in carrying out diagnoses, in order to avoid the dissemination and proliferation of the disease in Brazilian cattle herds, contributing to minimizing the damage and losses suffered by producers, whether directly or indirectly.
2. Methodology
To prepare this study, a cross-sectional and exploratory survey was carried out in existing bibliographies on VDVB, and these works were selected from the scientific databases Scielo, Medline, Google Scholarship and Lilacs, in which a cross-sectional analysis and a retrospective survey were carried out to collect data from research carried out by several authors in various regions of the country, on the seroprevalence, etymological origin of VDVB, as well as the factors that predispose animals to the risk of contagion, prophylactic measures and damages caused to livestock farmers due to the disease, where publications carried out from 1987 to the present day were analyzed.
Regarding the population survey on the number of animals in the country's cattle herds, a survey was carried out according to the data provided by IBGE updated in the year 2023 and brought and described in the study.
From the data collected, an evaluative and careful analysis was made in the selected works and transferred to the current research, where articles that addressed the topic in question were introduced in this study, being transcribed in a descriptive manner and with a qualitative approach, seeking to highlight and indicate the average and relative rate of seroprevalence existing in Brazilian cattle herds, in addition to pointing out the risk factors that predispose to the emergence of new cases of contamination and spread of infectivity in the herd. Regarding the inclusion and exclusion process, these were determined based on the proposal that guides the research, in which all articles that contained data relevant to this work were included.
3. Results And Discussion
Infectious diseases that cause the death of embryos and fetuses are responsible for more than 50% of the reproductive problems that affect cattle farming, with BVD, Leptospirosis and Infectious Bovine Rhinotracheitis (IBR) being among the main contagious diseases that affect the reproductive tract of animals. However, BVDV is considered the most relevant, as it presents several symptoms, such as lesions in the oral and intestinal cavity, pneumonia, nasal discharge, gastroenteritis, immunosuppression and teratogenicity, which can be evidenced through serological tests [2, 32,49, 51]. Bovine Viral Diarrhea Virus (BVDV) has great genomic and antigenic heterogeneity. They are small and spherical, have icosahedral and globular symmetry, measuring between 40 and 60 nm in diameter. They are an enveloped virus with a genome formed by single-stranded RNA and positive chain, belonging to the Flaviviridae family, of the Pestivirus genus. They are capable of stipulating two forms of infection, one transient and the other persistent. The disease affects most bingulated animals, and can infect cattle, buffaloes, small ruminants, pigs, among other animals; however, cattle are the most susceptible to infection [3,15, 23, 42,51].
Despite their virulence, virions are stable at pH levels between 5.7 and 9.3 and are easily inactivated by organic solvents such as chloroform and ether, common disinfectants and detergents such as chlorhexidine, iodophors, hypochlorites, aldehydes and phenols, and can be kept frozen for years at -70ºC or in a lyophilized state. However, they do not remain in the environment for more than 15 days [8, 50]. According to GASPARINI, M. R et al.[24], due to the pathogenicity of VDVB, the disease is of great importance and concern for cattle farming, generating major economic impacts for producers in the country, since, in addition to causing respiratory and gastrointestinal problems, it causes conception failures, causing infection of the uterus, and enabling an increase in the number of calves born infected by the persistent form of the disease (PI), responsible for maintaining the agent in the herd, as they eliminate viral particles through excretions and secretions throughout their lives, these being the main source of spread of the virus [18, 24, 33].
Viral pathogenesis depends on several conditions, such as the nutritional, health and physical status of the animals. In addition, certain hosts can influence contamination and damage caused by the infection, such as in the case of immunosuppressed animals, the age of the animal, transplacental contamination, stressful conditions, among other factors [32,51].
BVDV can be eliminated through excretions and secretions of infected animals, such as nasal discharge, eye secretion, urine, saliva and milk [12]. Viral penetration occurs through the oral and nasal routes, multiplying in the lymphoid tissue of the mouth, pharynx and tonsil epithelial cells. After multiplication, it advances to the bloodstream through the lymphatic vessels. High viral concentrations are present in the spleen, lymph nodes, salivary glands and respiratory tract. The first lesions occur in the upper respiratory tract, in the gastrointestinal tract and lymphatic system of affected animals [15, 32,51].
Due to mutations and structural rearrangements that occur in the E2 glycoproteins, BVDV has a high antigenic variability, determining the occurrence of two important genotypes, BVDV1 and BVDV2, divided into several subtypes. BVDV-1 is related to the classic infection and has 11 subgroups, while BVDV-2, which has a higher degree of virulence, has only two subtypes: A and B. Their genotypes can be distinguished through genetic research and by administering antibodies against the viral proteins E2 and ERNS [8,15,23]. Furthermore, BVDV has two biotypes, the cytopathogenic (CP) which causes a cytopathic effect in cell cultures, leading to cell death, and the non-cytopathogenic (NCP) which is more important, as it is the only one capable of crossing the placental membrane and invading the fetus, causing persistent contamination in it. Furthermore, the NCP does not cause any evident modification in cell cultures, making its identification in vitro difficult [3, 8, 15,23].
The CP biotype originates from mutations, deletions and genetic rearrangements through duplication or insertion of the virus genome into the non-structural viral protein NS2/3, which activates cleavage, giving rise to a new protein, NS3, with the capacity to cause cell damage, present only in this biotype. However, most contaminations caused by BVDV are due to the non-cytopathogenic biotype. The presence of BVDV in both strains may present mild or inapparent signs [3, 38,50].
The antibodies acquired by animals that were infected by the acute form of the disease protect cattle from a new co-infection, which is deferred for the rest of their lives [33,38]. Infected animals without pregnancy, they may present mild symptoms, such as fever, respiratory and gastrointestinal problems and leukopenia, in addition to causing a drop in milk production and a decline in weight gain in production animals, both dairy and beef. However, the agent's survival mechanism in cattle herds is due to contamination that affects pregnant females that are vulnerable to the virus, due to its capacity for transplacental transmission [33, 42, 46].
The damage caused by infection to fetuses depends especially on the gestation period at which contamination occurs. Infections contracted in the early stages of gestation can cause females to return to estrus due to embryonic reabsorption [33]. Acute contamination in pregnant cows between 40 and 120 days of gestation can result in vertical transmission of the NCP strain, resulting in the birth of PI animals. In addition, it can result in a drop in the reproductive index, such as embryonic loss, abortions, malformations, stillbirths, mummified fetuses, and the birth of nonviable and weak animals, when the infection occurs at a more advanced stage of gestation [12,33, 38, 42,46].
In male animals, when infected by the acute form of the disease, it can cause a decrease in semen motility and density, increasing the number of sperm and reducing the fertility rate, causing testicular hypoplasia, in addition to intense viral replication in them. It is worth highlighting the fact that PI animals are crucial factors in the epidemiology and maintenance of this disease, being considered one of the main aggravating factors in the control of DVD, as they do not present symptoms of the disease, being the main disseminators and consequently causing most cases to go unnoticed, being diagnosed only after viral spread throughout the herd [2, 32].
Infections caused by viral agents are a source of ongoing concern for any type of health planning in cattle farming. Correct diagnosis requires epidemiological knowledge and the use of appropriate laboratory tests to identify and combat the causative agent [3]. In Brazil, the disease was first identified through serological evaluations in the early 1970s in the Rio Grande do Sul region [23]. The main means of identifying the BVD agent is through the serum neutralization test. In addition to SN, animals can also be evaluated through other diagnostic mechanisms, such as the virus neutralization test, which is based on neutralization of the agent, and the ELISA test, which is a serological method used to detect antibodies against the BVD virus in the animals analyzed. Methods such as polymerase chain reaction and virus isolation in cell culture have also been used directly [12,49].
BVDV has two structural glycoproteins (E2 and ERNS) and one non-structural glycoprotein (NS2-3) of greater importance, capable of inducing the humoral immune response and aiding in viral diagnosis. The immunodominant glycoprotein E2 (previously called gp56) is responsible for the induction and formation of host neutralizing antibodies. It has a transmembrane visible on the outside of the viral cell, which forms heterodimers with E1 (E1-E2) and homodimers (E2-E2). It is considered the most stable protein, used to aid in the diagnosis and classification of viral serotypes. The ERNS glycoprotein has activity in the RNAse enzyme, which acts by degrading the RNA of contaminated cells and secreting small fractions into the extracellular medium, contributing to the persistence of BVDV. It also induces and forms partially attenuated antibodies, aiding in viral detection, and can be found in the tissues and serum of contaminated cattle. NS2-3 is a well-conserved protein with high immunogenicity, being used mainly for diagnosis [3,13, 29].
In Brazil, BVD is an endemic disease and is considered enzootic, with an average prevalence of 60 to 85% of animals reactive to infection, and is widespread in several regions of the country [2.5, 49,50].
Ribeiro et al [45], in 1987, conducted a study in the region of Bahia, in which they evaluated 1,618 animals using the serum neutralization test (SN), in which 14.64% of these animals tested positive for the infection. In Pernambuco, Castro, Roberto S et, in 1993 [9], conducted an epidemiological study using viral NADL for sampling, in which samples from 288 cattle were analyzed using the SN method in microplates, and demonstrated that 209 of these cattle were reactive to the infection, presenting a prevalence of 72.64%. In the state of São Paulo, LANGONI, H. et al [28], in 1995 analyzed 184 samples through the ELISA test, and observed a prevalence of 39.5%, while in the analysis carried out by PITUCO, E. M.; DEL FAVA, C., [39] in 1998, in SP, positivity was noted in 40.80% of the samples, from the 493 animals evaluated by SN. In 1997, KRAHL, M. et, [27] and FIGUEIREDO, H. C. P. et al. [20], carried out their studies using the SN method, in which they presented a seroprevalence of in the state of RS (23.40% reagents of the 1,823 animals tested) and in MG (61.47% reagents of the 287), respectively.
CANAL, Cláudio Wageck et al. [7], in 1998, evaluated samples from 430 adult animals from 19 properties in the state of RS using the ELISA test, observing antibodies in 56% of these cattle. It is worth noting that the test used has 99.2% specificity and 97.5% sensitivity. In Paraná, MÉDICI, K. C. et al. [30], analyzed 937 samples using SN, where they found 73.52%, in the year 2000. In the study carried out in 2002 by BRITO, W. M. E. D et al [6], in the state of Goiás, in which 452 animals were tested using the ELISA method, 35.2% presented antibodies. Studies that were carried out during the period from 1987 to 2002 in relation to the seroprevalence of BVD in cattle herds, in which animals from various properties in different regions of the country were evaluated, demonstrated that the average prevalence rate varied between 14.64% and 73.52% of the animals are reactive, according to the period and region analyzed, as shown in table 1.
|
Region |
References |
Animals |
Assay |
% Prevalence / Nº of positive |
|
Bahia |
Ribeiro et al., 1987 |
1.618 |
SN |
14,64 (237) |
|
Pernambuco |
Castro et al., 1993 |
288 |
SN |
72,64 (209) |
|
São Paulo |
Langoni et al., 1995 |
184 |
ELISA |
39,5 (73) |
|
Rio Grande do Sul |
Krahl et al., 1997 |
1.823 |
SN |
23,40 ( |
|
Minas Gerais |
Figueiredo et al.,1997 |
287 |
SN |
61,47 ( |
|
São Paulo |
Pituco et al., 1998 |
493 |
SN |
40,8 ( |
|
Rio Grando do Sul |
Canal et al., 1998 |
430 |
ELISA |
56 ( |
|
Paraná |
Médici et al., 2000 |
937 |
SN |
73,52 ( |
|
Goiás |
Brito et al., 2002 |
452 |
ELISA |
35,2 ( |
|
Overall |
--- |
6.512 |
--- |
2.408 Reagents (37%) |
|
|
||||
Table 1: Analysis of the seroprevalence of BVDV infection in cattle herds, carried out in various regions of Brazil from 1987 to 2002.
When analyzing Table 1, it was noted that during the period from 1987 to 2002, 6,512 animals were analyzed, coming from various properties and regions of the country, of which 2,408 were reactive for the infection, indicating a relative prevalence and morbidity of 37%, calculated through the total number of reactive animals over the total number of animals analyzed. Thus, it can be stated that in the researched period, it demonstrated that for every 100 animals tested, 37 animals were reactive for BVD in the various regions of the country analyzed, according to the sample N of this analysis.
However, other highly relevant studies carried out during the same period analyzed 2,447 samples of cattle from 56 properties without vaccination, 34 of which were for dairy production and 21 for meat. These animals were subjected to serological examination through the indirect ELISA test and showed a seroprevalence of 83.67% in the region of Mato Grosso do Sul, 71% in Rio de Janeiro, 67% in Paraná, 65% in Minas Gerais, 73% in Rio Grande do Sul and 78% in the state of São Paulo. This study observed that all properties had at least 1 cattle that tested positive for the disease, and that 57% of these farms had a seroprevalence greater than 75%, suggesting widespread viral spread in the regions analyzed [47]. In another study carried out near Aparecida de Goiânia, the prevalence found was 54.11%, where they evaluated 207 samples collected between the end of 1997 and 1998 [25].
Serological analyses carried out in Brazil between 1971 and 2004 showed the presence of antibodies against BVDV in herds from various regions of the country, such as the Central-West, Southeast, South and Northeast. The high seroprevalence rate of this antigen highlights concerns about the identification and knowledge of risk factors related to infection and the recommendations for prophylactic measures proposed to protect the country's cattle herds [11,34]. In the most recent studies carried out between 2003 and 2021, the results of serological surveys carried out to detect BVD in cattle herds in different regions of the country demonstrated that the average seroprevalence rate of the infection in the animals analyzed varied between 22.20 and 99.60%, as can be seen in table 2, which shows the data according to the author, period in which the research was carried out, the state, type of tests used, number of animals analyzed and the seroprevalence obtained of the disease in the country's herds.
|
Region |
References |
Animals Analyzed / |
Assay |
Nº of properties analyzed / |
|
Bahia |
Noronha et al., 2003 |
220 / 56 / 123 |
SN |
8 / 100% / 8 |
|
Rio Grande do Sul |
Poletto et al., 2004 |
204 / 29,41 / 60 |
ELISA |
28 / 67,85% / 19 |
|
South Minas Gerais Northeast São Paulo Total |
Samara et al., 2004 |
245 / 57,56 / 141 131 / 56,49 / 74 376 / 57,18 / 215 |
ELISA |
10 / 100% / 10 (from 12,28 to 100%) |
|
Paraíba |
Thompson et al., 2006 |
2.343 / 22,20 / 520 |
SN |
72 /88,90% / 64 |
|
Rio Grande do Sul |
Quincozes et al., 2007 |
1.734 / 66,32 / 1.150 |
SN |
85 / 82,35% / 70 (from 14,29 to 100%) |
|
Minas Gerais |
Mendes et al., 2009 |
126 / 71,42 / 90 |
ELISA |
--- / --- / --- |
|
Minas Gerais Mato Grosso do Sul São Paulo Total |
Ribeiro, 2009 |
519 / 71,87 / 373 499 / 99,60 / 497 482 / 47,51/ 229 1.500 / 73,20 / 1.099 |
VN |
15 / -- / -- 5 / -- / -- 5 / -- / -- 20 / -- / -- |
|
Several municipalities Goiás |
De Brito et al., 2010 |
3.533 / 64 / 2.261 |
SN |
888 / 88,30% / 784 |
|
Minas Gerais/ São Paulo |
Alexandrino et al., 2011 |
278 / 69,78 / 194 |
VN |
6 / 94,74% / -- |
|
Several municipalities Maranhão |
Chaves et al., 2012 |
920 / 65,66 / 604 |
ELISA |
92 / 94,57% / 84 |
|
Maranhão |
De Sousa et al., 2013 |
156 / 67,30/ 105 |
ELISA |
16 / 100% / 16 (from 20 to 100%) |
|
Rio Grande do Sul |
Piovesan et al., 2013 |
2.763 / 85,40 / 2.359 |
SN |
157 / -- / -- |
|
São Paulo |
Silva, 2014 |
12.854 / 47,08 / 6.051 |
VN |
1.723 / 78,21% / 1.354 |
|
Maranhão |
Abas, 2014 |
396 / 54,04 / 214 |
ELISA |
33 / 90,91% / 30 |
|
Paraíba |
Tolentino et al., 2014. |
352 /40,9 / 144 |
ELISA |
20/ 95% /19 |
|
Maranhão |
Sousa, 2017 |
364 / 64,56 / 235 |
ELISA |
12 / 100% / 12 (from 5 to 95,23%) |
|
Minas Gerais |
Nasciutti et al., 2017 |
264 / 45,1 / 119 |
VN |
20 / -- /-- |
|
Rio Grande do Sul |
Duarte et al., 2018 |
66 / 59,10 / 39 |
ELISA |
2 / 100% / 2 |
|
Pernambuco |
Pajeú et al., 2019 |
357 / 99,4 / 355 |
VN |
18 / 100% / 18 (from 93,5 to 100%) |
|
Minas Gerais |
Augusto et al., 2021 |
123 / 70,73 / 87 |
ELISA |
-- / -- / -- |
|
Overall |
------- |
28.929 / 55,39 / 16.024 |
----- |
3.168 / 78,57% / 2.489 |
|
-- Data not provided by the source |
||||
Table 2: Distribution of seroprevalence of BVDV infection in cattle herds, carried out in different regions of Brazil between 2003 and 2021.
During the time period of these 18 years studied, a total of 28,929 animals were examined, from 3,168 properties distributed in different regions of the country, in which, of these tested animals, 16,024 presented antibodies for BVD, coming from 2,489 herds, as shown in Table 2. In view of this, it was possible to observe that the relative seroprevalence rate and morbidity of the disease in Brazilian herds showed that 55.39% of the animals have antibodies for the infection. Where it can be concluded that for every 100 animals examined, 55 are reactive for BVD, according to the sample N, where the total number of cases is evaluated over the total number of the population analyzed.
When comparing the data in Table 1, where the time period analyzed was between 1987 and 2002, with Table 2, carried out with more recent studies, a considerable increase in the morbidity rate of the disease was noted, since in the period of Table 1 this was around 37%, while in the period studied in Table 2 the morbidity presents a rate of 55.39% of seropositivity in the animals. This can be justified due to the fact that nowadays there has been a significant increase in the number of animals tested for BVD, if comparing the number of animals examined between the period of Table 1 in relation to the number of animals tested in the period of Table 2, this probably occurred due to the greater ease and feasibility found for carrying out serological tests in the current times. Regarding the spread of BVD in herds, it was possible to observe that the frequency rate of seropositivity of the disease for the animals examined per property varied between 5 and 100%, demonstrating that in practically all the properties examined there was at least one animal reactive to the disease. Where it was possible to observe a morbidity of 78.57% within the properties studied. In view of this, it is possible to note that possibly the source of contagion is internally within the properties themselves, and that it does not come only from the acquisition of new animals in the herd [25].
Serological surveys carried out between 2003 and 2021 demonstrated a high seroprevalence rate in several regions of Brazil, in which the study indicated that the highest prevalence occurred in the South region of the country, where a rate of 75.68% was found, while in the Central-West region the rate found was 64%, in the Southeast it was 50.60%, while in the Northeast region of the country it found a prevalence of 45.02% of animals reactive for BVD, as shown in graph 1.
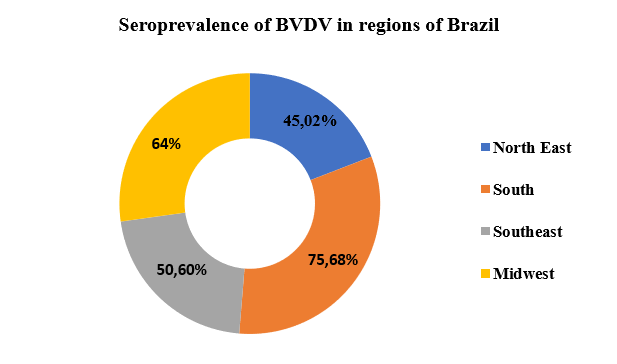
Graph 1: Seroprevalence of VDVB in the regions of the country, according to the data found by the authors.
When analyzing graph 1, the result found in this research is in agreement with the work previously carried out by Dezen et al. [11] in 2013 and Nasciutti et al. [34], in 2017, where they pointed out the high presence of the infection in the herds of these regions. This was evidenced by the present study, which demonstrated that even today there is still a high dissemination and high prevalence rate of the infection in these regions of the country.
In view of this, it is clear that conducting epidemiological studies and surveys on the prevalence of BVD are extremely important, due to the severity of the infection, the high morbidity rate and seroprevalence of the disease in cattle farming, in addition to the economic problems caused by the disease to livestock farmers. Since the knowledge existing in the literature regarding the etymological origin and occurrence of reproductive infections is still not satisfactory, given such severity. In view of this, several studies are being carried out with the aim of seeking a better understanding of the pathogenicity and prophylactic measures, with the purpose of preventing and combating viral contamination and spread in cattle herds, in addition to seeking to assist owners and technical managers in seeking more efficient prophylactic measures, since, to date, there is no effective form of control sanctioned for its containment [2, 5, 49,50].
Another factor that impacts disease control is related to early diagnosis of the agent's circulation on the property, as well as the culling of PI animals. Vaccination in herds is essential to aid in control and prophylactic measures, however, the vaccine only reaches a small part of the herd. Another problem encountered in vaccine prevention is due to the difficulty in performing serological tests on the herd and because it consists only of acute immunization of the disease, this lack of effectiveness is due to the high variability of existing strains [32].
Due to the wide range of symptoms of the infection, it is necessary to perform a differential diagnosis with other diseases, in addition to creating a program for control, monitoring and prevention of BVD. On farms that use insemination methods, it is important to carry out sanitary control of semen, since these are considered another important source of dissemination. For more effective control of the disease, it is essential to take several prophylactic measures, such as quarantining new animals, controlling the movement of animals between properties, adopting biosecurity measures with hygiene and management, constant monitoring of herds, among others. It is extremely important for farmers to be aware of the severity of the disease, since there is no specific treatment for the infection and it is a disease with high morbidity and spread [5, 21,32, 37].
4. Conclusion
With the help of this study, we conclude that BVD is a disease that affects the reproductive system and is of great concern to cattle producers. Its presence causes several financial losses, directly or indirectly. It is an endemic disease in Brazil, where it has high seroprevalence rates in animals. Through this study, it was possible to see that 55.39% of these animals have antibodies for the infection. In addition, we can observe that Brazilian herds have a high rate of morbidity and viral spread, where it was observed that the seroprevalence of the disease within the properties is 78.57%. In addition, through the serological surveys carried out, it was possible to see high rates of infection present in herds in the South, Center-West, Southeast and Northeast regions. In view of this, we conclude that more effective prophylactic measures need to be taken, such as the disposal of PI animals and constant monitoring of herds, in order to reduce the spread and incidence of the disease in new animals.