International Journal of Epidemiology And Public Health Research
OPEN ACCESS | Volume 9 - Issue 1 - 2026
ISSN No: 2836-2810 | Journal DOI: 10.61148/2836-2810/IJEPHR
Cassava Effluent and Testicular Toxicity: A Laboratory Investigation
Gabriel d. Edem1*, Michael A. Amadi2, Samuel J. Umanah3, Abasifreke S. Okon1
1Department of Human Anatomy, Faculty of Basic Medical Sciences, University of Uyo, Uyo, Nigeria.
2Department of Anatomy, Faculty of Basic Medical Sciences, PAMO University of Medical Sciences, Port Harcourt, Nigeria.
3Department of Human Anatomy, Faculty of Basic Medical Sciences, Novena University, Delta State, Nigeria.
*Corresponding author: Gabriel D. Edem, Department of Human Anatomy, Faculty of Basic Medical Sciences, University of Uyo, Uyo, Nigeria.
Received: April 15, 2025
Accepted: April 25, 2025
Published: April 28, 2025
Citation: Gabriel d. Edem, Michael A. Amadi, Samuel J. Umanah, Abasifreke S. Okon. (2025) “Cassava Effluent and Testicular Toxicity: A Laboratory Investigation”. International Journal of Epidemiology and Public Health Research, 6(3); DOI: 10.61148/2836- 2810/IJEPHR/124
Copyright: © 2025. Gabriel D. Edem. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited., provided the original work is properly cited
Introduction: This research was carried out to investigate the relationship between cassava effluent and testicular toxicity. Testes are male sex organs that possess both exocrine and endocrine functions. They are oval-shape reproductive structures that are found in the scrotum and separated by the scrotal septum. Cyanide is a fast acting potentially toxic chemical that can exist as both gas and crystalline salt.
Methods: Twenty (20) adult male Wistar rats weighing between 115-205g were used for this study and the experiment lasted for 28 days. The rats were divided into five groups of four rats each namely; group I (control), group 2, group 3, group 4 and group 5. Rats in these groups were given normal diet/distilled water, 20ml/kg Garri effluent, 10ml/kg Garri effluent, 20ml/kg of Fufu effluent and 20ml/kg of Fufu effluent respectively. At the end of the last dose schedule, the rats were sacrificed and blood sample obtained through the inferior vena cava for hormonal assay. Testicular tissues were processed histologically and stained using H&E.
Results: At the end of this research, the result showed that the serum levels of testosterone, follicle stimulating hormone (FSH) and luteinizing hormone (LH) were significantly reduced. Several histological alterations such as proliferation of atypical germ cells, degenerated connective tissue, necrotic spermatids and few/absence of spermatozoa within the seminiferous tubules associated with the administration of cassava effluent were noticed
Conclusion: Cassava effluent is highly acidic due to the presence of cyanide. Care must be taken during the consumption of this staple food product in most part of the world in order to prevent damage to the reproductive organs.
Introduction:
Cyanide is a fast acting and potentially deadly chemical that can exist as both a gas and a crystalline salt. Both forms can be lethal in high concentrations. It is formed abundantly in nature as well as in man-made materials produced from industries. They are very common in the environment due to the natural and human generated sources and people are exposed to cyanide routinely at low air concentration throughout the environment. Cyanide can cause significant changes in body and reproductive organ weight, sperm motility, sperm count, sperm abnormality and the epididymis [1, 2]. The most toxic cyanides are hydrogen cyanide (HCN), sodium cyanide (NaCN), potassium cyanide (KCN) and calcium cyanide (Ca(CN)₂) [2]. These compounds are extremely poisonous and require careful handling to avoid severe health risks. The cyanide anion is an inhibitor of the enzyme cytochrome c oxidase which is the fourth complex of the electron transport chain found in the inner membrane of the mitochondria of eukaryotic cells. It attaches to the iron within this protein. The binding of cyanide to this enzyme prevents transport of electrons from cytochrome c to oxygen. As a result, the electron transport chain is disrupted, meaning that the cell can no longer aerobically produce ATP for energy [3]. Tissues that depend highly on aerobic respiration, such as the central nervous system and the heart, are particularly affected. This is an example of histotoxic hypoxia [4]. The most hazardous compound is hydrogen cyanide, which is a gas which kills by inhalation [5]. Hydrogen cyanide is produced by adding acid to a solution containing a cyanide salt. Hydrogen cyanide may be produced in the combustion of polyurethane and for this reason, polyurethanes are not recommended for use in domestic and aircraft furniture [4]. Oral ingestion of a small quantity of solid cyanide or a cyanide solution as little as 200mg, or exposure to airborne cyanide of 270ppm, is sufficient to cause death within minutes [4]. Although cyanide levels in cassava tubers can be reduced using simple traditional methods such as boiling, roasting, drying, grating, soaking and fermentation, consumption of raw cassava tubers is being practiced probably due to lack of awareness of potential dangers of cyanide poisoning [6, 7]. Furthermore, local classification of cassava varieties as “sweet” (i.e., non-poisonous) has added to the complacency in employing the simple treatments to reduce cyanide levels in tubers before consumption. This is because even those cassava cultivars considered sweet have been a source of disaster to humans in many parts of the world [8].
Materials and Methods:
Garri effluent processing method:
Cassava tubers were gotten from a local farm in a local community of Akwa Ibom State, Nigeria. They were peeled, washed, pulverized and processed the same day so that it could be suitable for Garri. It was also poured in a fenestrated bag after pulverization and pressed under iron for extraction of water generally referred to as Garri effluent.
Fufu effluent processing method:
Cassava tubers were gotten from a local farm in a local community of Akwa Ibom State, Nigeria. They were peeled, washed and soaked in tap water to undergo fermentation and later pulverized at the cassava mill and poured into a fenestrated bag and pressed under iron in order to extract some water known as Fufu effluent.
Animal care and protocol:
Twenty male Wistar rats weighing between 115 – 205g were obtained from the Faculty of Basic Medical Sciences’ Animal house, University of Uyo, UYo, Nigeria. The animals were weighed, labeled and kept in plastic cages and fed with vital feeds and water. The animals were divided into four (4) animals per cage of 5 groups according to the method described by [1].
Acclimatization of experimental animals:
The animals were housed in plastic cages and acclimatized for a period of two weeks in the Animal House of the Faculty of Basic Medical Sciences, University of Uyo. Acclimatization took place in order to help them get used to their new surroundings throughout the duration of the study. The animals were given unlimited access to food and water.
Intervention and administration of cassava effluent:
Cassava effluents were administered orally accordingly to the method described by Edem et al. [1] for 28 days. The animals were categorised into 5 groups which include;
Group 1 (control group): given normal diet and tap water
Group 2: given 20ml/kg Garri effluent.
Group 3: given 10ml/kg Garri effluent.
Group 4: given 20ml/kg Fufu effluent.
Group 5: given 10ml/kg Fufu effluent.
Sacrifice and serum collection:
At the end of twenty-eight (28) days, all animals were sacrificed and blood was collected via the inferior vena cava for hormonal assay. Testicular tissues were harvested and immediately fixed in 10% neutral buffered formalin.
Tissue processing:
The testes were placed in 10% neutral buffered formalin after the sacrifice, in order to preserve the organs, prevent enzymes autolysis, post-mortem changes and bacterial decay. They were histologically processed and stained using H&E.
Microscopy:
Processed tissues were viewed under the light microscope and photomicrographs were obtained using the microscope’s camera attached to a personal computer.
Statistical analysis:
Data obtained from the study were expressed as mean ± standard error of mean and were analysed using one-way analysis of variance (P<0.05).
Results:
Effect of cassava effluent on testosterone, FSH and LH serum levels:
Result showed decreased testosterone level in the experimental animals especially in group 2 when compared to the control group. It was also observed that LH and FSH levels were significantly decreased in a dose-dependent manner.
|
Groups |
Testosterone (ng/ml) |
LH (mlu/mL) |
FSH (mlu/mL) |
|
Control |
0.773± 0.013 |
7.414± 0.243 |
9.030± 0.814 |
|
20/ml/kg GE |
0.439±0.009** |
6.189±0.610** |
8.380± 1.363** |
|
10/ml/kg GE |
0.566± 0.010* |
5.976± 0.458* |
7.843±1.400 |
|
20/ml/kg FE |
0.504± 0.027** |
5.199± 0.671** |
7.186± 0.773** |
|
10/ml/kg FE |
0.599± 0.017* |
6.100± 0.503* |
6.834± 0.559* |
Table 1: Effect of cassava effluent on testosterone, FSH and LH
Values are express in mean ± standard error of mean
* = significantly different from control and other groups @ P<0.05
** = significantly different from control, group 2 and group 5@ P<0.05
Histomorphological effect of cassava effluent on the testes
The histological section of group 1 (control) animals given water and feed alone as represented in figure 1 shows normal spermatogonia, primary spermatocytes, interstitial cell of Leydig, connective tissue, spermatids and seminiferous tubules with the presence of densely packed spermatozoa. In figure 2, the histological section revealed proliferation of atypical germ cells within the seminiferous tubules, degenerated connective tissue, necrotic spermatids and few/absence of spermatozoa within the seminiferous tubules. Figure 3 revealed necrotic spermatids, degenerated connective tissue, Leydig cell necrosis, necrosis of spermatogenic cells and deformed seminiferous tubule with very scanty spermatozoa.
Figure 4 revealed necrotic spermatids, vacuolation in the region of the connective tissue septum, necrosis of spermatogenic cells and presence of very scanty spermatozoa within the deformed seminiferous tubules. Similarly, Figure 5 revealed sclerosed sertoli cells arranged in tight cords and clusters, necrotic spermatids, degenerated connective tissue and presence of very scanty spermatozoa within the deformed seminiferous tubule. All the sections from the experimental groups differ from the control group section.
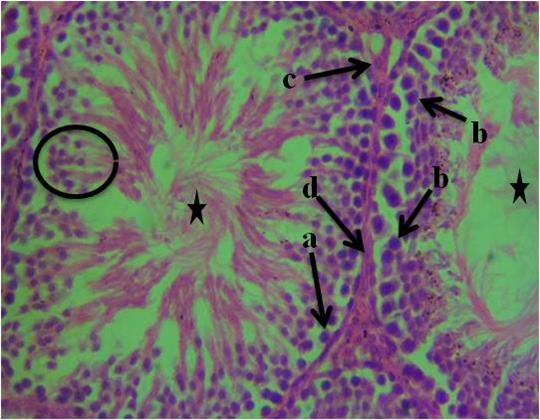
Figure I: Photomicrograph of testis of control animals given water and feed alone showing, a- spermatogonia, b-primary spermatocytes, c-interstitial cell of Leydig, d-connective tissue, spermatids (circle) and seminiferous tubules with the presence of densely packed spermatozoa (star). (H&E). 400x magnification.
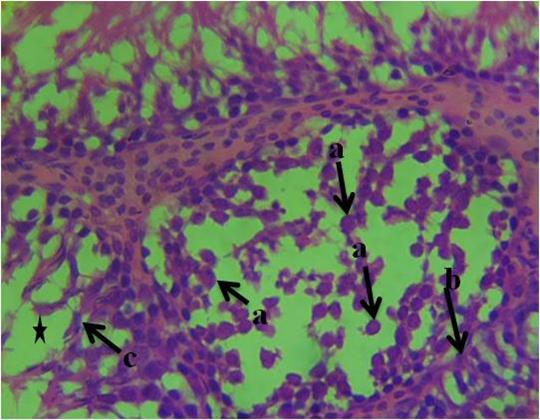
Figure 2: Photomicrograph of testis of group 2 animals given 20ml/kg of Garri effluent showing, a-proliferation of atypical germ cells within the seminiferous tubules, b-degenerated connective tissue, c-necrotic spermatids and few/absence of spermatozoa within the seminiferous tubules (star). (H&E). 400x magnification.
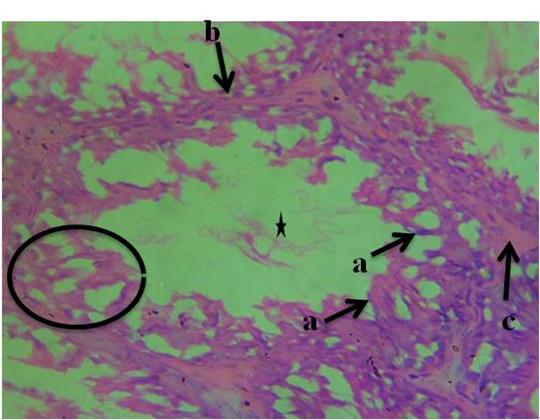
Figure 3: Photomicrograph of testis of group 3 animals given 10ml/kg of garri effluent showing, a-necrotic spermatids, b-degenerated connective tissue, c-Leydig cell necrosis, necrosis of spermatogenic cells (circle) and deformed seminiferous tubule with very scanty spermatozoa (H&E). 400x magnification
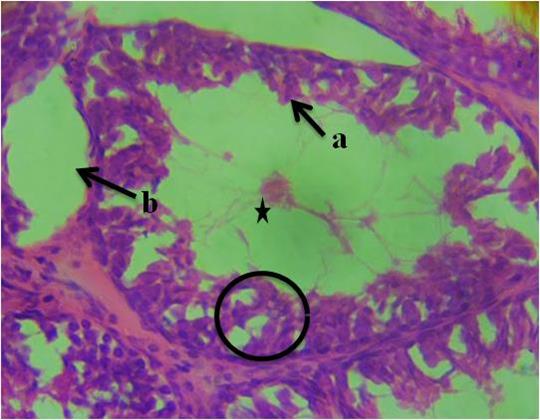
Figure 4: Photomicrograph of testis of group 4 animals given 20ml/kg of fufu effluent showing, a-necrotic spermatids, b-vacuolation in the region of the connective tissue septum, necrosis of spermatogenic cells (circle) and presence of very scanty spermatozoa within the deformed seminiferous tubules (star). (H&E). 400x magnification.
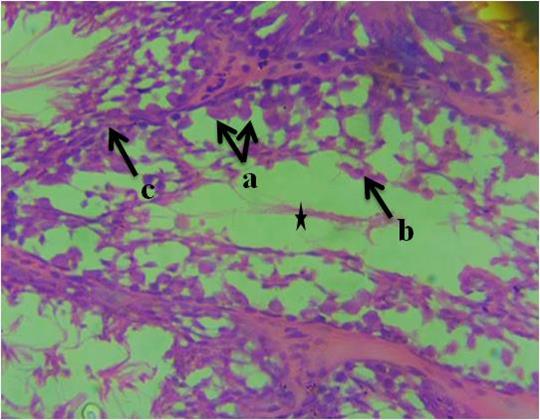
Figure 5: Photomicrograph of testis of group 5 animals given 10ml/kg of fufu effluent showing, a-sclerosed sertoli cells arranged in tight cords and clusters, b-necrotic spermatids, c- degenerated connective tissue and presence of very scanty spermatozoa within the deformed seminiferous tubule. (H&E). 400x magnification
Discussion:
The testis is highly sensitive to the oxidative damages caused by free radicals and testicular germ cells are very susceptible to oxidative stress due to their functional reproductive role [9]. Several factors such as pH homeostasis is very crucial for spermatogenesis, sperm maturation, sperm physiological function, and fertilization. HCO3− and H+ are the most significant factors involved in regulating pH homeostasis in the male reproductive system. Oxidative stress is a major cause of endocrine disruption and since the underlying mechanism in cyanide toxicity is oxidative stress, cyanide induced toxicity can therefore result in hormonal disruption [10]. Oxidative stress affects particularly the hypothalamic-pituitary-gonadal (HPG) axis, which regulates reproductive hormones [10]. The disruption of this axis by cyanide toxicity can lead to alterations in the levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), and testosterone in males, affecting reproductive health and function. This report is in tandem with our study which revealed decreased serum levels of the aforementioned hormones. This may be due to the fact that, cyanide exposure can cause oxidative stress and damage to hypothalamic neurons, leading to reduced secretion of gonadotropin-releasing hormone (GnRH). Consequently, this reduction in GnRH can result in decreased levels of FSH and LH in the bloodstream. Oxidative stress caused by cyanide can further impair testosterone synthesis by damaging the mitochondria in Leydig cells, which are crucial for steroidogenesis [11]. The combined effect of reduced LH and direct oxidative damage can lead to significant declines in testosterone levels. Our result in table 1 showed that testosterone, FSH and LH levels all decreased after administration of cassava effluent when compared to the control. Li et al.[11] reported a decrease in the serum levels of testosterone, luteinizing and follicle stimulating hormones due to cyanide induced toxicity. This is similar to our results where Garri effluent caused the most significant decrease in testosterone level and the least decrease in FSH and LH level. This could be due to the fact that the secretion of FSH and LH is regulated by testosterone in a negative feedback manner [12]. So a lower testosterone level would cause a higher FSH and LH level as observed in the experimental animals. Our result differed from the submission of Yang et al. [13] who reported increase in FSH and LH levels in rats with atrazine-induced toxicity due to reduced inhibin level, a complimentary hormone in feedback regulation of FSH and LH. This contrast in the result could be due to the level of toxicity or dosage between the toxicants.
Results from our histological sections revealed the proliferation of atypical germ cells within the seminiferous tubules, degenerated connective tissue, necrotic spermatids and few/absence of spermatozoa within the seminiferous tubules. This result is in tandem with that of Ceribasi et al. [14] who observed that cyclosphomide-induced toxicity in the testes of rats appeared to have necrotic sperm cells, degeneration of the connective tissue, disorganization and reduction in germinal cells and atrophy in tubules due to induced oxidative stress which causes lipid peroxidation. Their findings are consistent with most alterations present in figure 2. Furthermore, our results also revealed alterations such as necrotic spermatids, degenerated connective tissue, Leydig cell necrosis, necrosis of spermatogenic cells and deformed seminiferous tubule with very scanty spermatozoa. The evidence of Leydig cell necrosis, deformation of the seminiferous tubule and scanty spermatozoa is consistent with the findings of Christian et al. [15] who reported similar evidence due to cadmium-induced toxicity. They attributed the presence of scanty spermatozoa due to the deformation of seminiferous tubule which is the area for sperm production and necrosis of Leydig cells which is responsible for testosterone secretion necessary for spermiogenesis. Other histological evidence revealed in our work include; necrotic spermatids, vacuolation in the region of the connective tissue septum, necrosis of spermatogenic. The presence of vacuolation as observed is similar to those found in the testicular toxicological study by [16] using alcohol-induced toxicity which caused oxidative stress. According to [17] reactive oxygen species (ROS) can induce oxidative stress, leading to lipid peroxidation and subsequent vacuolation in the testis. It can damage the cellular components, including the membranes of the cells in the connective tissue, leading to the formation of vacuoles [17]. Appearance of sclerosed Sertoli cells arranged in tight cords and clusters, necrotic spermatids, degenerated connective tissue and presence of very scanty spermatozoa within the deformed seminiferous tubule also supported the toxicological impact of cassava effluent. The presence of sclerosed Sertoli cells arranged in tight cords and clusters is consistent with the findings of Hew et al. [18] who reported that exposure to induced oxidative stress can disrupt the tight junctions between Sertoli cells and alters Sertoli–germ cell adhesion. The seminiferous tubules, Sertoli cells, Leydig cells and other areas of the testes are essential for reproductive function [19]. Our results clearly show that cassava effluent due to its cyanide content has severe impact on the histoarchitecture of the testes therefore implicating it functions. Based on the histological alterations, Garri effluent and Fufu effluent presented no much distinct difference but Garri effluent was found to affect the Leydig cells more thereby affecting testosterone levels.
Conclusion:
In conclusion, this study revealed that cassava effluent has serious deleterious effect on testicular histostructure and hormonal balance as a result of its cyanide content which can have adverse implications on reproductive health.
Statement of Financial Support: None
Conflicts of Interest: None