Gastroenterology and Hepatology Research
OPEN ACCESS | Volume 6 - Issue 1 - 2025
ISSN No: 2836-2888 | Journal DOI: 10.61148/2836-2888/GHR
Ndjitoyap Ndam Antonin Wilson¹, ²*, Shu Sonia Charlsia Ewuo³, Maimouna Mahamat²,⁴, Dang Babagna Isabelle¹,⁵, Talla Paul¹, Kowo Mathurin², Ankouane Andoulo Firmin², Ashutantang Gloria Enow²,³,⁴
¹Hepato gastroenterologist service, Yaoundé general hospital, Yaoundé, Cameroon.
²Internal medicine department, Faculty of Medicine and Biomedical sciences, University of Yaoundé 1, Yaoundé, Cameroon.
³Department of clinical sciences, Faculty of Health sciences, University of Bamenda, Bamenda, Cameroon.
⁴Nephrologist service, Yaoundé general hospital, Yaoundé, Cameroon.
⁵Hepato gastroenterologist service, Centre Medical la Cathedrale, Yaoundé, Cameroon.
*Corresponding author: Ndjitoyap Ndam Antonin Wilson, Hepato gastroenterologist service, Yaoundé general hospital, Yaoundé, Cameroon.
Received date: October 14, 2023
Accepted date: October 23, 2023
Published date: October 27, 2023
Citation: Ndjitoyap Ndam Antonin Wilson, Shu Sonia Charlsia Ewuo, Maimouna Mahamat, Dang Babagna Isabelle, Talla Paul.et al.. (2023) “ Factors associated with renal impairment in Patients on Tenofovir for Chronic Hepatitis B in Yaoundé (Cameroon)”, J of Gastroenterology and Hepatology Research, 4(1); DOI: 10.61148/2836-2888/GHR/040.
Copyright: © 2023 Ndjitoyap Ndam Antonin Wilson. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background: Tenofovir (TFV) is widely used to treat patients with hepatitis B virus (HBV) infection. But kidney abnormalities are the main concern using this drug. Few studies have described the renal impairment due to the TFV in chronic hepatitis B (CHB) in Sub-Saharan Africa. The objective was to evaluated factors associated with renal impairment observed in patients on TFV for CHB. Method: It was a hospital based cross sectional study carried out from June 2023 to July 2023 in Yaoundé (Cameroon). Was included any patient treated with TFV for CHB during at least a period of 6 months. For each participant, we collected socio-demographic data, clinical data, baseline creatinine, treatment information (type of TFV which was Disoproxil Fumarate (TDF) or Alafenamide (TAF), duration). Then, we collected blood samples to measure serum creatinine and phosphate levels and urine dipstick analysis. Factors associated with renal impairment were assessed with the Odds Ratio. A p value of < 0.05 was significant. Results: A total of 60 participants were included. The median age was 44 years [36-55] and median duration of TFV therapy was 17.5 months [11.7-25.7]. The prevalence of reduced eGFR (<60ml/min/1.73m2) was 6/60 (10%), the prevalence of hypophosphatemia 6/60 (10%) and the prevalence of albuminuria 24/60 (40%). Factors associated with eGFR reduced were diuretic use (OR 8.5, [1.09-9.58], p=0.042) and duration of TFV≥36 months (OR 34, [4.3 – 266.3], p=0.001). Those associated with hypophosphatemia was duration of TFV≥36 months (OR 12.5 [1.88 – 83.3], p=0.009). While factors associated with albuminuria were TDF prodrug use (OR 8.8 [1.8 – 43.1], p=0.009), and duration of TFV≥36 months (OR 11.7 [1.3 – 104.5], p=0.009). Conclusion: Kidney function was impaired in some patients receiving TFV for CHB. It should be monitored, particularly after 36 months and for those receiving TDF or diuretics.
Background:
Hepatitis B is a deadly inflammation of the liver caused by the hepatitis B virus (HBV) [1]. Untreated, the infection could lead to liver cirrhosis or hepatocellular carcinoma. The prevalence in Cameroon is estimated to 11.2%, varying from one region to another [2]. Tenofovir (TFV) is an antiviral widely used in monotherapy to treat patients with hepatitis B virus (HBV) infection [3-5]. It could be the Tenofovir Disoproxil Fumarate (TDF) at the posology of 300 mg once daily, other the Tenofovir Alafenamide (TAF) 25 mg per day [1]. This last seems have a lower nephrotoxicity than the first one. The TFV is also prescribed in against HIV in an anti-retroviral treatment regimen [6-7]. But kidney abnormalities are the main concern using this drug. Hypophosphatemia is a possible complication in patients. The TFV is associated with a risk of proximal tubular dysfunction and declining estimate glomerular filtration rate (eGFR) [8-10]. Fanconi syndrome is a possible adverse reaction of TFV treatment, especially in HIV-infected patients [11]. It leads to excessive urinary excretion of solutes handled by the proximal tubule, such as phosphate, glucose, and bicarbonate. Age ≥60 years, diabetes mellitus, high blood pressure, and high serum bilirubin have also been described as risk factors for the development of renal insufficiency in chronic hepatitis B (CHB) patients receiving TDF therapy [12]. But, the mechanism of renal impairment in patients with HVB is multifactorial. In addition to the antiviral therapy nephrotoxicity, the kidney disease can be due to the virus itself [13]. The commonest type is membranous glomerulonephritis. Therefore, the monitoring of renal function is recommended during treatment [3,14]. If there are studies describing renal impairment associated with the use of TFV against HIV in sub-Saharan Africa, few studies have described its effects on renal function in CHB in our area [15-17].
Objective:
The objective was to identify factors associated with renal impairment in patients on TFV for CHB.
Materials And Method:
A hospital based cross sectional study was carried out over a period of 2 months (June 2023 to July 2023) in two referral hepato gastrointestinal units of the Cameroonian capital: Yaoundé General Hospital and Centre Médical la Cathédrale (Yaoundé, Cameroon). Was included consenting patient treated with TFV for CHB during at least 6 months. Patients whose baseline creatinine was not recorded at the start of the treatment were excluded. For each participant, we collected socio-demographic data (age, sex), clinical data (BMI, blood pressure readings at rest, comorbidities), baseline biological characteristics (creatinine, Alanine aminotransferase (ALAT) and Aspartate aminotransferase (ASAT)), treatment (type of TFV which was TDF or TAF, duration of treatment and associated treatment). Then, we collected blood samples to measure serum creatinine (to calculated the eGFR) and phosphate levels by spectrophotometry, and midstream urine for dipstick urine analysis.
Blood sample and urine analysis:
All samples collected for serum creatinine were centrifuged and stored in the hospital’s freezer at -20°C for at least one week. We analyzed the samples ourselves using a spectrophotometer (HUMAN®). Creatinine was determine using the Jaffe reaction and the GFR is later calculated using the CKD-EPI formula.
All samples collected for serum phosphorus were centrifuged and stored in the hospital’s freezer at -20°C. We analyzed the samples ourselves using a spectrophotometer (HUMAN®)
The principle is based on the fact that inorganic phosphate reacts with ammonium molybdate in the presence of sulfuric acid to form a phosphomolybdic complex which is measured at 340 nm. The absorbance at this wavelength is directly proportional to the amount of inorganic phosphorus present in the sample. The procedure described by the fabricant was respected.
Urine analysis was realized using a dipstick LABSTIX®. Holding the dipstick at the end opposite to the chemical pads, we dipped it into the freshly collected urine sample for approximately 2 seconds. The dipstick was compared with the colour chart on the dipstick container and each parameter read after its recommended time.
Data Management and Analysis Plan:
At the end of daily data collection, completed forms were assessed, validated, coded, and stored. Data was entered Access Microsoft and exported Excel. The information was stored on a computer and on an external drive with a password fixed to maintain confidentiality and safety. Data was analyzed according to objectives using the Statistical Package for Social Sciences (SPSS) version 26 and Microsoft Excel.
Renal abnormalities studied were a reduced of eGFR (<60ml/min/1.73m2), a hypophosphatemia (Serum phosphorus <2.5mg/dL), and an albuminuria (more than 1+ proteinuria on dipstick). Factors associated with renal impairment were assessed with the Odds Ratio (OR). A p-value of < 0.05 was considered significant after bivariate analysis.
Results:
Two hundred and seven (207) files of participants on TFV treatment for CHB over five years were retrieved. After implementing our inclusion criteria, eighty-six participants were eligible to participate in the study for which we had access to sixty of them.
Socio demographic characteristics of the study population:
Of the 60 participants, 68.3% (n=41) were males and the median age [IQR] was 44.0 [36-55] years (table 1)

Table 1: Socio demographic characteristics of the study population.
Comorbidities in the study population:
Hepatitis D (20 patients, 33.3%), obesity (18 patients, 30%), hypertension (12 patients, 20%), and diabetes mellitus (7 patients, 11.7%) were the most frequent comorbid conditions. We also observed liver cirrhosis (5 patients, 8.3%), HIV (3 patients, 5%), an underweight with BMI< 18 Kg/m2 (3 patients, 5%) and a hepatocellular carcinoma (1 patient, 1.7%).
CHB treatment:
The median [IQR] duration of TFV therapy was 17.5 [11.7-25.7] months. We noted 53 patients with duration of TFV ≥36 months. TDF was the main prodrug observed in 42 patients while TAF was noted in 18 patients.
Other medications used in the study population:
Herbal African medication (21.7%), angiotensin converting enzyme inhibitors (13.3%) and metformin (11.7%) were the most frequent drugs used. We also noted diuretics (8.3%), angiotensin receptor blocker (8.3%), and non-steroidal anti-inflammatory drugs (6.7%).
Baseline biological characteristics of the study population:
Of the 60 participants, only 1.7% (1 patient) had a raised serum creatinine at baseline. Until 30 patients (50%) and 29 patients (48.3%) had raised ALAT and ASAT at baseline respectively. We registered 26 patients (43.3%) with Hepatitis B viral load>2000 IU/ml.
Renal function of the population at time of the study:
After at least 6 months of TFV treatment, 6 patients (10%) of the participants had an eGFR<60ml/min/1.73m2. There was an increase in mean serum creatinine from baseline (0.95mg/dl) to after at least 6 months of TFV treatment (1.05mg/dl) with change in mean serum creatinine being 0.1mg/dL (p=0.005) (figure 1). There was a decrease in mean eGFR from baseline (93.2ml/min/1.73m2) to after at least 6 months of TFV treatment (83.6ml/min/1.73m2) with change in mean eGFR being 9.6ml/min/1.73m2 (p=0.001) (figure 1). All 6 participants who had an eGFR <60ml/min/1.73m2 were between the ranges 45-59ml/min/1.73m2.
The median [IQR] serum phosphorus was 3.3[2.8-3.8] and a total of 6 participants (10%) had hypophosphatemia.
A total of 24 participants (40%) had albuminuria and 5 (20%) were nephrotic range albuminuria (more than 300 mg/dl).
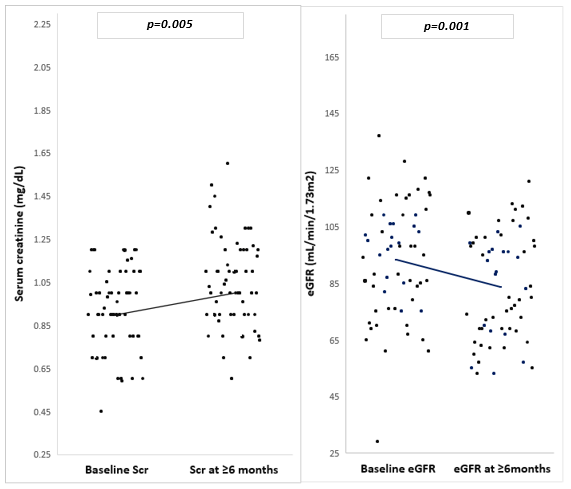
Figure 1: Changes in mean serum creatinine and eGFR of the study population (n=60).
Factors associated with reduced estimated glomerular filtration rate, hypophosphatemia and albuminuria:
In bivariate analysis, factors associated with eGFR reduced were diuretic use (OR 8.5 [1.09-9.58], p=0.042) and duration of TFV≥36 months (OR 34 [4.3 – 266.3], p=0.001) (table 2).
Factors associated with hypophosphatemia was duration of TFV≥36 months (OR 12.5 [1.88 – 83.3], p=0.009) (table 3).
While factors associated with albuminuria were TDF prodrug use in contrast with TAF prodrug use (OR 8.8 [1.8 – 43.1], p=0.009), and duration of TFV≥36 months (OR 11.7 [1.3 – 104.5], p=0.009) (table 4).
|
Variable |
Categories |
Reduced eGFR |
OR (95% CI) |
p-value |
|
|
Yes(n=6) |
No(n=54) |
||||
|
n(%) |
n(%) |
||||
|
Age (Years)
|
<60 |
2(33.3) |
11(20.4) |
1 |
0.471 |
|
≥60 |
4(66.7) |
43(79.6) |
1.96[0.32-12.0] |
||
|
Sex
|
Male |
3(50) |
38(70.4) |
0.42[0.08-2.31] |
0.320 |
|
Female |
3(50) |
16(26.9) |
1 |
||
|
Medication
|
ARB use |
0(0) |
5(8.4) |
Undefined |
0.999 |
|
ACEI use |
2(33.3) |
6(11.1) |
4.00[0.60-26.68] |
0.152 |
|
|
NSAIDS use |
1(16.7) |
3(5.6) |
3.40[0.30-39.10] |
0.326 |
|
|
Diuretics use |
2(33.3) |
3(5.6) |
8.50[1.09-6.58] |
0.042 |
|
|
Herbal med use |
1(16.7) |
12(22.2) |
0.70[0.07-6.58] |
0.755 |
|
|
TFV prodrug |
TDF |
5(83.3) |
37(40.7) |
2.30[0.25-21.2] |
0.463 |
|
TAF |
1(16.7) |
17(31.5) |
1 |
||
|
Comorbidities |
Hypertension |
2(33.3) |
10(18.5) |
2.20[0.35-13.7] |
0.399 |
|
Diabetes |
0(0) |
7(13) |
Undefined |
0.999 |
|
|
HDV |
3(50) |
17(31.5) |
2.18[0.40-1.92] |
0.370 |
|
|
BMI (kg/m2) |
< 18 |
1(16.7) |
2(3.7) |
5.20[0.40-7.94] |
0.209 |
|
≥ 18 |
5(83.3) |
52(96.3) |
1 |
||
|
Duration of TFV therapy(months) |
<36 ≥ 36 |
4(66.7) 2(33.3) |
3(5.6) 51(94.4) |
1 34.00[4.34-266.3] |
0.001 |
|
ALAT, IU/L |
>36 |
3(50) |
27(50) |
1.00[0.19-5.40] |
1.000 |
|
≤36 |
3(50) |
27(50) |
1 |
||
|
ASAT, IU/L |
>35 |
3(50) |
26(48.1) |
1.08[0.20-5.82] |
0.931 |
|
≤ 35 |
3(50) |
28(51.9) |
1 |
||
|
Hepatitis B viral load, IU/ml |
>2000 |
3(50) |
23(43.4) |
1.30[0.24-7.07] |
0.78 |
|
≤2000 |
3(50) |
30(56.6) |
1 |
||
Table 2: Factors associated with reduced estimated glomerular filtration rate (bivariate analysis) (n=60)
ACEI=angiotensin converting enzyme inhibitor, ALAT=alanine aminotransferase, ARB= angiotensin receptor blocker, ASAT=aspartate aminotransferase, BMI= body mass index, NSAIDS=non-steroidal anti-inflammatory drugs, TAF=tenofovir alafenamide, TDF=tenofovir disoproxil fumarate, TFV=tenofovir.
|
Variable |
Categories |
Hypophosphatemia |
OR[95% CI] |
p-value |
|
|
Yes(n=6) n(%) |
No(n=(54) n(%) |
||||
|
Age (Years)
|
<60 |
3(50) |
10(18.5) |
1 |
0.095 |
|
≥60 |
3(50) |
44(81.5) |
4.4[0.77-25.10] |
||
|
Sex |
Male |
4(66.7) |
37(68.5) |
0.92[0.15-5.51] |
0.926 |
|
Female |
2(33.3) |
17(31.5) |
1 |
||
|
Medication |
ARB use |
0(0) |
5(9.3) |
Undefined |
0.999 |
|
ACEI use |
1(16.7) |
7(13.0) |
1.34[0.14-13.25] |
0.801 |
|
|
Diuretics use |
1(16.7) |
4(7.4) |
2.50[0.23-26.91] |
0.450 |
|
|
Herbal med use |
2(33.3) |
11(20.4) |
1.95[0.32-12.09] |
0.471 |
|
|
TFV prodrug |
TDF |
6(100) |
36(66.7) |
Undefined |
0.998 |
|
TAF |
0(0) |
18(33.3) |
1 |
||
|
Comorbidities |
Hypertension |
1(16.7) |
11(20.4) |
0.78[0.08-7.39] |
0.830 |
|
HDV |
4(66.7) |
16(29.6) |
4.75[0.79-28.60] |
0.089 |
|
|
BMI (kg/m2) |
< 18 |
1(16.7) |
2(3.7) |
5.20[0.40-67.94] |
0.209 |
|
≥ 18 |
5(83.3) |
52(96.3) |
1 |
||
|
Duration of TFV therapy(months) |
<36 |
3(50) |
4(7.4) |
1 |
0.009 |
|
≥ 36 |
3(50) |
50(92.6) |
12.50[1.88-83.31] |
||
|
ALAT,IU/L
|
>35 |
3(50) |
27(50) |
1.00[0.19-5.40] |
1.000 |
|
≤35 |
3(50) |
27(50) |
1 |
||
|
ASAT,IU/L
|
>36 |
3(50) |
26(48.1) |
1.08[0.20-5.82] |
0.931 |
|
≤ 36 |
3(50) |
28(51.9) |
1 |
||
|
Hep B Viral load, IU/ml |
>2000 |
2(33.3) |
24(45.3) |
0.60[0.10-3.59] |
0.579 |
|
≤2000 |
4(66.7) |
29(54.7) |
1 |
||
Table 3: Factors associated with hypophosphatemia (bivariate analysis) (n=60).
ACEI=angiotensin converting enzyme inhibitor, ALAT=alanine aminotransferase, ARB= angiotensin receptor blocker, ASAT=aspartate aminotransferase, BMI= body mass index, NSAIDS=non-steroidal anti-inflammatory drugs, TAF=tenofovir alafenamide, TDF=tenofovir disoproxil fumarate, TFV=tenofovir.
|
Variable |
Categories |
Albuminuria |
OR(95% CI) |
p-value |
|
|
|
|
Yes(n=24) |
No(n=36) |
|
|
|
|
|
n(%) |
n(%) |
|
|
|
Age (Years)
|
<60 |
8(33.3) |
5(13.9) |
1 |
0.081 |
|
≥60 |
16(66.7) |
31(86.1) |
3.10[0.87-11.04] |
||
|
Sex
|
Male |
14(58.3) |
27(75) |
0.47[0.15-1.41] |
0.320 |
|
Female |
10(41.7) |
9(25) |
1 |
||
|
Medication |
ARB use |
|
|
1.00[0.15-6.48] |
1.000 |
|
ACEI use |
5(20.8) |
3(8.3) |
2.90[0.62-13.48] |
0.176 |
|
|
Metformin use |
2(8.3) |
5(13.9) |
0.56[0.10-3.17] |
0.516 |
|
|
NSAIDS use |
2(8.3) |
2(5.6) |
1.56[0.23-11.79] |
0.326 |
|
|
Diuretics use |
3(12.5) |
2(5.6) |
2.43[0.37-15.58] |
0.352 |
|
|
Herbal med use |
8(33.3) |
5(13.9) |
3.10[0.87-11.04] |
0.081 |
|
|
TFV prodrug |
TDF |
22(91.7) |
20(55.6) |
8.80[1.80-43.15] |
0.007 |
|
TAF |
2(8.3) |
16(44.4) |
1 |
||
|
Comorbidities |
Hypertension |
7(29.2) |
5(13.9) |
2.55[0.70-9.29] |
0.155 |
|
Diabetes |
2(8.3) |
5(13.9) |
0.56[0.10-3.17] |
0.516 |
|
|
HDV |
7(29.2) |
13(36.1) |
0.73[0.24-2.22] |
0.577 |
|
|
BMI (kg/m2) |
< 18 |
2(8.3) |
1(2.8) |
3.18[0.27-37.94] |
0.356 |
|
≥ 18 |
22(91.7) |
35(97.2) |
1 |
||
|
Duration of TFV therapy |
<36
|
6(25)
|
1(2.8)
|
1
|
0.028 |
|
≥ 36 |
18(75) |
35(97.2) |
11.7[1.30-104.53] |
||
|
ALAT, IU/L
|
>36 |
8(83.3) |
5(13.9) |
1.32[0.49-3.72] |
0.598
|
|
≤36 |
11(45.8) |
19(58.2) |
1 |
||
|
ASAT, IU/L
|
>35 |
12(50) |
17(47.2) |
1.18[0.40-3.12] |
0.833
|
|
≤ 35 |
12(50) |
19(52.8) |
1 |
||
|
Hep B Viral load, IU/ml |
>2000 |
12(50) |
14(40) |
1.50[0.54-4.27] |
0.448
|
|
≤2000 |
12(50) |
21(60) |
1 |
||
Table 4: Factors associated with albuminuria (bivariate analysis) (n=60)
ACEI=angiotensin converting enzyme inhibitor, ALAT=alanine aminotransferase, ARB= angiotensin receptor blocker, ASAT=aspartate aminotransferase, BMI= body mass index, HDV=hepatitis D virus, NSAIDS=non-steroidal anti-inflammatory drugs, TAF=tenofovir alafenamide, TDF=tenofovir disoproxil fumarate, TFV=tenofovir.
Discussion:
We included 60 patients receiving TFV for CHB since at least 6 months. We observed a male predominance with a median age of 44 years. This male has also been described in Cameroon by Halle et al in 2019 [13]. The explanation is probably due to higher risk of HBV and onset of a hepatocellular carcinoma much elevated in men than in women [18]. Our population was younger than those described by Jung et al in 2018 in Seoul (South Korea) [12]. African patients seem to develop hepatocellular carcinoma younger than those in Asia and West countries [19]. For this reason, in South Saharan Africa we like to prescribe antiviral treatment against HBV early than in Asia and West.
Mean changes in creatinine:
At the time of the study, we registered 6 patients (10%) with an elevated serum creatinine in contrast with only one patient before the treatment. This will induce a poor eGFR which is estimated through the serum creatinine. The changes show that the kidney injury is due to the antiviral treatment and not due the HBV itself. This result in inferior to 25.4% observed by Yazie et al in 2018 in Ethiopia [10]. This last study was conducted in a population of HIV-patients receiving TFV in combination with other antiretroviral therapy. For this reason, the poly medication could increase the risk of renal injuries.
Renal impairment:
We observed 6 participants (10%) who had an eGFR <60ml/min/1.73m2. The ranges were between 45-59ml/min/1.73m2. This value corresponds to a mild kidney injury but which becomes severe later [3]. A hypophosphatemia was observed in 6 participants (10%). This could be associated with a proximal tubular dysfunction leading to excessive urinary excretion of phosphate. Concerning albuminuria, it was observed in 24 participants (40%) including 5 with a nephrotic range albuminuria (more than 300 mg/dl). This abnormality is result in glomerular lesions due to the TFV treatment [8]. Without the diagnosis and treatment, this could induce a chronic kidney disease.
Factors associated with renal impairment:
Duration of TFV therapy ≥36 months were associated with a reduced eGFR OR=34.00 [4.34-266.3] p=0.001; with hypophosphatemia OR = 12.50 [1.88-83.31] p=0.009 and albuminuria OR = 11.7[1.30-104.53] p=0.028. Diuretics use was associated with eGFR OR = 8.50 [1.09-6.58], p= 0.024; and TDF prodrug use was associated with albuminuria OR = 8.80 [1.80-43.15], p=0.007. In contrary with some studies, old age, hypertension, diabetes mellitus and high viral load were not associated with kidney injuries in our population [12].
Limitations of current study:
Despite our important results, our study has some limitations. We wish to increase the size of the sample with a multicentric analysis with few loss of view. Moreover, our patients have a different duration of treatment. We wish to realize a prospective study to have data of all patients at the same time of treatment. The last limitation is the absence of a multivariate analysis to exclude confusing factors.
Conclusion:
Kidney function was impaired in some patients receiving TFV for CHB. It should be monitored, particularly after 36 months and for those receiving TDF or diuretics.
Declarations Ethics Approval:
Ethical clearance number 2023/0779H/UBa/IRB was obtained from the Institutional Review Board of the University of Bamenda (Bamenda, Cameroon). After clear explanation of the study, risk and benefits, only participants who gave their consent were included in the study.
Administrative authorization:
Before the recruitment, we obtained the administrative authorization from the Centre Regional Delegation of Public Health, the general director of the Yaoundé General Hospital and the director of the Centre Médical la Cathédrale.
Consent To Participate:
We approached all the patients of our target population and explained the aim and procedure of the study to them. We further explained the risk and benefits of the study to them. We then requested their consent to participate in the study. Those who accepted to participate gave their consent either verbally or signed the consent form.
Author contributions:
Ndjitoyap Ndam Antonin Wilson:designed the study protocol, wrote the manuscript.
Shu Sonia Charlsia Ewuo: investigator, collected data, wrote the manuscript.
Maimouna Mahamat: analyzed data.
Dang Babagna Isabelle: collected data.
Talla Paul: collected data.
Kowo Mathurin: analyzed data.
Ankouane Andoulo Firmin: worked as supervisor.
Ashutantang Gloria Enow: worked as supervisor.
Competing Interests:
The authors state that there is no conflict of interest.
Funding:
The authors state that they did not receive any fund for their study.