Pharmacy and Drug Innovations
OPEN ACCESS | Volume 4 - Issue 1 - 2025
ISSN No: 2994-7022 | Journal DOI: 10.61148/2994-7022/PDI
Hari Prasad Sonwani
Department of Pharmacology, Assistant Professor, Apollo College of Pharmacy, Durg (Chhattisgarh) 491001, India.
*Corresponding author: Hari Prasad Sonwani, Department of Pharmacology, Assistant Professor, Apollo College of Pharmacy, Durg (Chhattisgarh) 491001, India.
Received: September 30, 2025 | Accepted: October 02, 2025 | Published: October 09, 2025
Citation: Hari Prasad Sonwani., (2025) “GPBA is a G-protein Coupled Receptor (GPCR) that Responds to Bile Acids and is Gaining Attention as A Potential Therapeutic Target for Disorders Related to Digestion and Sensation.” Pharmacy and Drug Innovations, 5(1); DOI: 10.61148/2994-7022/PDI/085.
Copyright: © 2025 Hari Prasad Sonwani. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Bile acids (BAs) are critical digestive agents essential for the emulsification and absorption of dietary lipids. Due to the pulsatile nature of BA secretion and their subsequent intestinal reabsorption, BA concentrations in circulation and tissues — much like gut hormone levels — fluctuate significantly between fasting and fed states. Moreover, BA profiles and abundance are profoundly altered under pathological conditions. Beyond their classical digestive roles, BAs function as signaling molecules by activating both nuclear and membrane-bound receptors. Nuclear steroid receptors mediate the genomic effects of BAs, regulating BA, glucose, and lipid metabolism. In contrast, GPBA (also known as TGR5), a G-protein coupled receptor located at the plasma membrane, orchestrates many of the rapid, non-genomic responses to BAs. GPBA has been implicated in the modulation of glucose homeostasis, inflammatory processes, and hepatic function. Emerging evidence also highlights a novel role for GPBA within the nervous system. GPBA is expressed in enteric neurons and enterochromaffin cells involved in the regulation of gut motility, where it mediates the well-recognized prokinetic effects of BAs. Furthermore, GPBA is found on primary sensory afferents and spinal neurons critical for sensory signal transmission. Activation of GPBA in sensory pathways by BAs induces pruritic behavior and analgesia, potentially explaining the itching and painless jaundice observed in patients with chronic cholestatic diseases characterized by elevated systemic BA levels. These insights underscore GPBA as a promising therapeutic target for a broad spectrum of metabolic, inflammatory, gastrointestinal, and sensory disorders, with potential applications for both receptor agonists and antagonists.
Bile acids (BAs), Nuclear steroid receptors, G-protein coupled receptor, homeostasis, inflammatory, non-genomic
G protein-coupled bile acid receptor 1 (GPBA), also known as TGR5, is a G protein-coupled receptor (GPCR) that responds to bile acids. Beyond its role in bile acid signaling, GPBA has gained attention as a key regulator of digestion and sensory pathways. Recent studies highlight its involvement in metabolic processes, gut motility, and the modulation of visceral sensation. As a result, GPBA is emerging as a promising therapeutic target for a range of digestive and sensory disorders. Understanding the mechanisms by which GPBA influences these functions could lead to new treatments for conditions such as irritable bowel syndrome, cholestatic diseases, and metabolic dysfunction, Bile acids (BAs) are essential components of bile, playing a critical role in the digestion and absorption of dietary fats through emulsification. In addition to their digestive functions, BAs act as important signaling molecules. Their levels in the bloodstream naturally fluctuate in response to physiological states and disease conditions, much like gut hormones. Through the activation of specific receptors located in the nucleus and on the plasma membrane, BAs influence a wide range of cellular processes throughout the body. These receptors allow BAs to regulate key physiological functions, including glucose metabolism and gastrointestinal motility, and are implicated in various pathological conditions, such as obesity and pruritus.
This review focuses on the G protein-coupled bile acid receptor (GPBA), also referred to as TGR5, GPBAR1, M-BAR, or GPR131, (Alexander et al., 2013). Over the past decade, research has demonstrated that GPBA is responsible for many of the rapid, non-genomic effects of BAs, influencing processes like thermogenesis, insulin release, glucose balance, inflammatory responses, digestive functions, and sensory signaling. Due to its broad physiological impact, GPBA is increasingly recognized as a promising therapeutic target for a variety of metabolic, inflammatory, digestive, and sensory disorders.
Bile Acids (BAs) as Hormone-like Signaling Molecules
Bile acids (BAs) function as signaling molecules with hormone-like effects. They form a large and complex family of amphipathic compounds characterized by a steroid nucleus. BAs show significant structural variation across different cells, tissues, physiological conditions, and between species. Although the full extent of BA complexity and diversity is beyond the scope of this article, it has been comprehensively reviewed (Hofmann and Hagey, 2008).
BAs are produced and broken down in mammalian cells and by gut microbiota through multiple, mostly well-understood enzymatic pathways (reviewed in Lefebvre et al., 2009). In the liver, hepatocytes synthesize BAs from cholesterol via a multi-step enzymatic process involving hydroxylation and shortening of the side chain. These modifications give BAs detergent-like properties essential for their main digestive role—emulsifying and promoting the absorption of dietary fats and fat-soluble vitamins (see Figure 1).
The primary bile acids synthesized in the liver include chenodeoxycholic acid (CDCA) and cholic acid (CA) in humans. Before being secreted into the bile ducts, primary BAs are conjugated with either taurine or glycine. Additional modifications, such as sulphation and glucuronidation, can occur in the liver.
BAs are stored in the gall bladder and released into the small intestine following meals, triggered by the hormone cholecystokinin, which is secreted by intestinal cells in response to dietary fats and induces gall bladder contraction.
Primary BAs are actively reabsorbed in the terminal ileum by the apical sodium-dependent bile acid transporter (ASBT) and the basolateral organic solute transporter complex (Ostα/Ostβ). Despite the efficiency of this absorption, a small fraction of BAs escape and reach the colon, where bacterial enzymes deconjugate, oxidize, and dehydroxylate them, producing secondary bile acids such as lithocholic acid (LCA) and deoxycholic acid (DCA) (see Figure 1). These bacterial modifications increase the hydrophobicity of BAs, enhancing their passive absorption through colonocytes.
The reabsorbed primary and secondary bile acids enter the hepatic portal circulation and return to the liver for reuse. This cycle of secretion, reabsorption, and recycling constitutes the enterohepatic circulation of bile acids, mainly involving CA, CDCA, and DCA.
Cholesterol (Liver)
↓ [CYP7A1, CYP8B1, others]
Primary Bile Acids (CA, CDCA)
↓ [Conjugation with Glycine/Taurine]
Conjugated Bile Acids
↓ [Secretion into Bile]
Bile (Gallbladder Storage)
↓ [Meal Stimulus]
Bile Acids released into Small Intestine
↓ [Microbial Action in Intestine]
Secondary Bile Acids (DCA, LCA)
↓
Reabsorption (Terminal Ileum)
↓
Portal Blood
↓
Liver (Recycling - Enterohepatic Circulation)
↓
Small Loss (~5%) in Feces
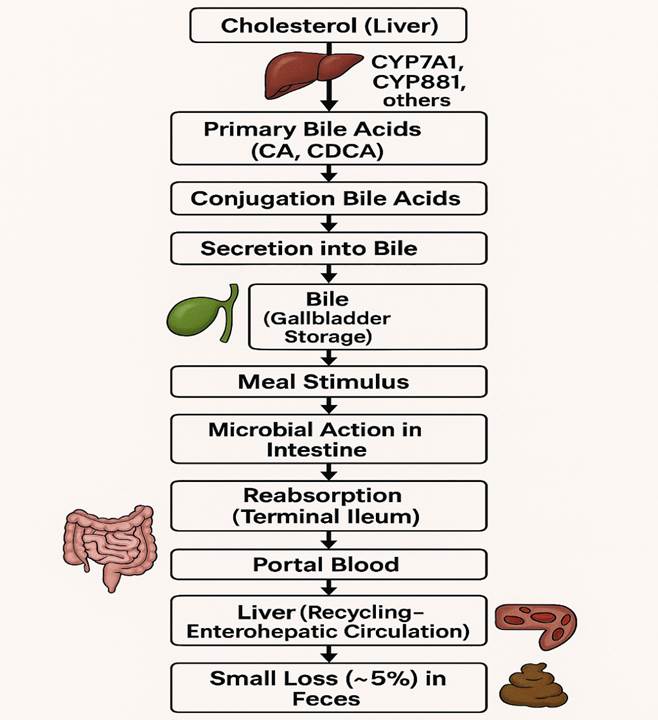
Figure 1:The synthesis, metabolism, and enterohepatic circulation of BAs.
The synthesis, transport, and secretion of bile acids (BAs) are tightly controlled by multiple physiological mechanisms that maintain their concentrations across different compartments and tissues, as well as regulate the overall composition of the BA pool. Because bile secretion occurs episodically, BA concentrations in the intestinal lumen, portal vein, and systemic circulation fluctuate between feeding and fasting, similar to patterns observed with gut hormones. Disruptions to the enterohepatic circulation, due to either disease or therapeutic interventions, can significantly alter both the concentration and composition of BAs within the intestine and bloodstream. For instance, in cholestatic diseases where bile acid secretion from the gallbladder is impaired, there is a reduction in BA delivery to the intestine alongside a substantial rise in circulating BA levels. These physiological and pathological changes likely influence the hormone-like functions of BAs.
Beyond their well-established role in solubilizing dietary fats and fat-soluble vitamins (A, D, E, and K), BAs act as signaling molecules that modulate cellular functions by activating specific receptors in both the nucleus and at the cell membrane. Among these, the nuclear receptors have been most extensively studied. Although a full discussion of these receptors is beyond the scope of this text, comprehensive reviews are available (Lefebvre et al., 2009). Nuclear receptors activated by BAs include the farnesoid X receptor (FXR), pregnane X receptor (PXR), and vitamin D receptor (VDR), all of which regulate gene expression in response to BAs. FXR, a ligand-activated transcription factor, is highly expressed in the liver, intestine, and kidney (Makishima et al., 1999; Parks et al., 1999; Wang et al., 1999). Primary and secondary BAs—specifically CDCA, LCA, DCA, and CA—can activate FXR, which plays critical roles in BA synthesis, detoxification, transport, and the regulation of lipoprotein metabolism and glucose balance. Additionally, LCA can activate PXR and VDR, contributing to cellular defenses against BA-induced toxicity (Staudinger et al., 2001; Xie et al., 2001; Makishima et al., 2002).
GPBA as a GPCR for Bile Acids (BAs)
Bile acids (BAs) are capable of modulating various G protein-coupled receptors (GPCRs) (Figure 2). For instance, conjugated forms of lithocholic acid (LCA) and deoxycholic acid (DCA) can influence muscarinic receptor activity in CHO and chief cells (Raufman et al., 1998; 2002). Additionally, DCA and chenodeoxycholic acid (CDCA) serve as antagonists for the formyl-peptide receptor (Le et al., 2002). Among these interactions, GPBA — also known as Gpbar1, M-BAR, or GPR131 — stands out as a genuine BA receptor (Maruyama et al., 2002; Kawamata et al., 2003). GPBA is encoded by a single exon gene, located on chromosome 1c3 in mice and 2q35 in humans. Its open reading frame encodes a 330 amino acid protein, characteristic of a seven-transmembrane domain GPCR, showing 28% similarity to the human sphingosine-1-phosphate receptor (EDG-1), a class A GPCR (Maruyama et al., 2002).
Several bile acids activate GPBA, but with varying strengths. In CHO cells expressing GPBA, the potency for stimulating cAMP production follows the order: taurolithocholic acid (TLCA, 0.33 μM) > LCA (0.53 μM) >> DCA (1 μM) > CDCA (4.4 μM) > cholic acid (CA, 7.7 μM), while ursodeoxycholic acid (UDCA) and cholesterol show minimal activity (Kawamata et al., 2003). Interestingly, oleanolic acid (OA), derived from the olive tree (Olea europaea), also activates GPBA with a potency similar to LCA but does not engage the farnesoid X receptor (Sato et al., 2007). A wide range of both steroidal and non-steroidal GPBA ligands have been explored for their therapeutic potential in metabolic and inflammatory diseases (Gioiello et al., 2012).
GPBA Expression Patterns
GPBA is broadly expressed across multiple tissues and cell types, though expression levels vary significantly. High GPBA mRNA expression is observed in the human liver, gastrointestinal tract, gallbladder, and immune tissues (Maruyama et al., 2002; Kawamata et al., 2003). Additionally, GPBA expression has been confirmed in gallbladder epithelial cells (Vassileva et al., 2006; Keitel et al., 2009), monocytes (Kawamata et al., 2003), sinusoidal endothelial cells, Kupffer cells (Keitel et al., 2007; 2008), brown adipose tissue, skeletal muscle (Watanabe et al., 2006), and neurons within the enteric nervous system, dorsal root ganglia, and central nervous system (Keitel et al., 2010a,b; Poole et al., 2010; Alemi et al., 2013b).
GPBA Signaling Mechanisms
In model systems like CHO and HEK cells, GPBA couples primarily to Gαs proteins, activating adenylyl cyclase and promoting cAMP production, which in turn stimulates protein kinase A (PKA) (Maruyama et al., 2002; Kawamata et al., 2003). There has been significant interest in the downstream effects of GPBA signalling, particularly its influence on cell proliferation and apoptosis in gastric and oesophageal cancers. For example, in human gastric carcinoma cells (ACS), DCA triggers prolonged ERK1/2 activation through a process that involves epidermal growth factor receptor (EGFR) transactivation, mediated by GPBA and the membrane protease ADAM17 (Yasuda et al., 2007).
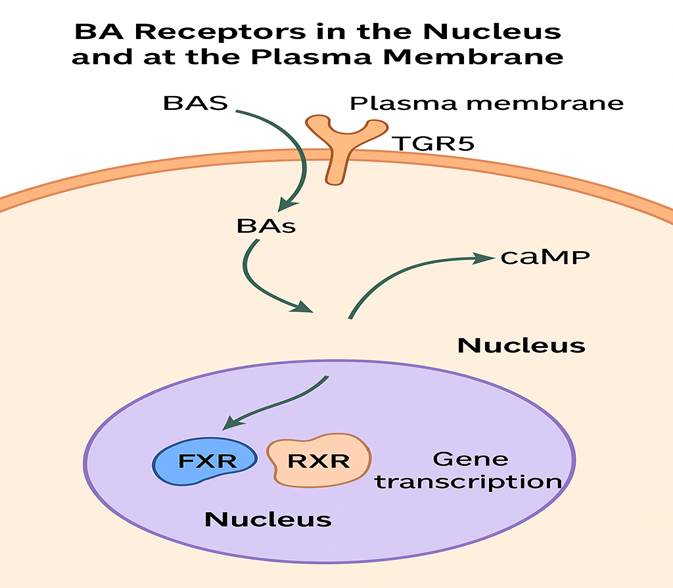
Figure 2 : BA receptors in the nucleus and at the plasma membrane.
Similarly, GPBA is upregulated in metastatic gastric adenocarcinomas, where taurodeoxycholic acid (TDCA)-induced proliferation of ACS cells depends on GPBA expression (Cao et al., 2013). In oesophageal adenocarcinoma cells (FLO), GPBA activation by TDCA induces NADPH oxidase NOX5-S expression, promoting reactive oxygen species generation and cell growth (Hong et al., 2010). Notably, in these cancer models, GPBA predominantly couples with Gαq proteins to drive BA-stimulated proliferation (Hong et al., 2010; Cao et al., 2013).
In liver cells, GPBA appears involved in bile acid-induced hepatocyte apoptosis, particularly during cholestasis. Knockdown of GPBA in human hepatocyte lines reduces bile acid-induced JNK activation, caspase 8 activation, and apoptosis, suggesting a pro-apoptotic role (Yang et al., 2007).
Anti-Inflammatory Roles of GPBA
GPBA also exhibits anti-inflammatory functions, particularly by suppressing macrophage activation (Pols et al., 2011). Activation of GPBA inhibits NF-κB-driven inflammatory signalling in activated B cells through a mechanism involving the interaction between IκBα and β-arrestin2 (Wang et al., 2011). In mice, deletion of GPBA worsens liver inflammation induced by lipopolysaccharides, underscoring its anti-inflammatory significance.
Regulation of GPBA Activity
Unlike many GPCRs, which undergo desensitization and internalization through β-arrestins and GPCR kinases, GPBA seems resistant to these regulatory mechanisms. Although GPBA activation leads to receptor internalization (Kawamata et al., 2003) and β-arrestin2 plays a role in some signalling contexts (Wang et al., 2011), detailed analyses reveal minimal direct interactions with β-arrestins or GPCR kinases (Jensen et al., 2013). Consistent with this, GPBA does not prominently traffic to endosomes, and cAMP signalling remains sustained without signs of rapid desensitization. Instead, activated GPBA relocates to lipid rafts, where it can interact with and transactivate the EGFR. Importantly, GPBA's anti-inflammatory effects are β-arrestin-independent (Pols et al., 2011). This resistance to desensitization may offer advantages in developing long-term GPBA-targeting therapies for metabolic or inflammatory diseases.
GPBA and Metabolic Regulation
Maintaining energy balance involves careful regulation of energy intake and expenditure. GPBA influences energy metabolism by activating type 2 iodothyronine deiodinase (D2), which converts inactive thyroxine (T4) into metabolically active triiodothyronine (T3) (Watanabe et al., 2006; Figure 3). Supplementing mice on a high-fat diet with cholic acid (CA) reduced fat accumulation, enhanced energy expenditure, and upregulated D2 expression in brown adipose tissue. These effects were absent in D2-deficient mice but persisted despite farnesoid X receptor inhibition, indicating a D2-mediated, GPBA-driven mechanism independent of nuclear BA receptors.
Further support for GPBA's role comes from studies where GPBA-specific agonists such as INT-777 increased energy expenditure and decreased body weight in wild-type and GPBA-overexpressing mice, but not in GPBA knockout mice (Thomas et al., 2009; Pols et al., 2011). Moreover, female GPBA knockout mice fed a high-fat diet exhibited greater body weight and fat content compared to wild-type controls (Maruyama et al., 2006), though other studies reported no significant changes (Vassileva et al., 2006). Thus, while a BA–GPBA–cAMP–D2 axis appears to influence energy metabolism, further research is needed to fully define GPBA's role in regulating energy expenditure and obesity.
GPBA and Glucose Homeostasis
GPBA and Bariatric Surgery
GPBA in Hepatic Inflammation and Injury
GPBA and Gallbladder Functions
GPBA in Intestinal Functions
GPBA and Genetic Associations
GPBA and Sensory Transduction
Overall, GPBA emerges as a key regulator in metabolism, liver protection, gut motility, gallbladder function, inflammatory responses, and sensory perception, making it a promising target for therapies in diabetes, liver diseases, gastrointestinal disorders, and cholestatic symptoms.
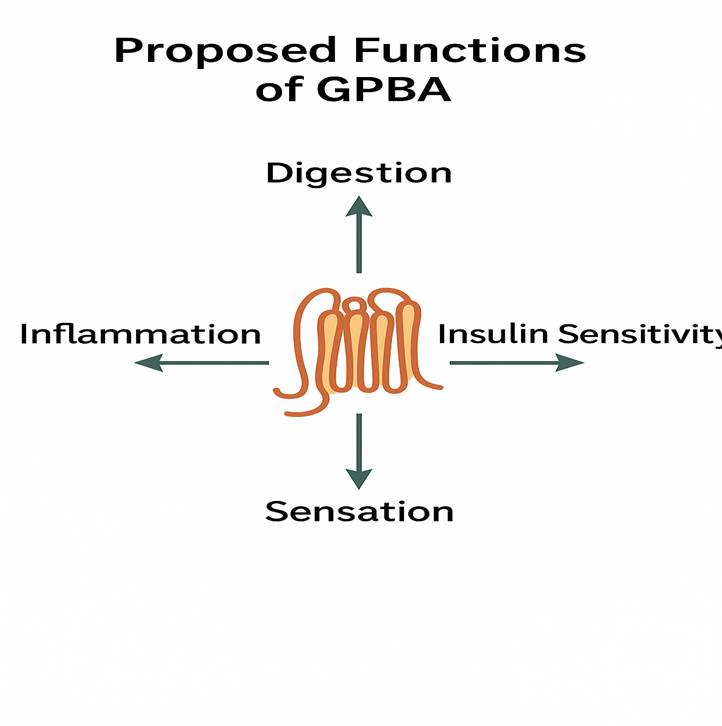
Figure 3 : Proposed functions of GPBA.
Conclusions and Future Perspectives:
GPBA (G-protein coupled bile acid receptor), also known as TGR5, has emerged as a promising therapeutic target for a range of metabolic diseases and disorders related to digestion and sensory functions. GPBA is widely expressed across various tissues, including the liver, intestine, brown adipose tissue, immune cells, and the nervous system, where it mediates a diverse array of physiological responses. Its pleiotropic effects, encompassing anti-inflammatory, digestive, metabolic, and sensory modulation pathways, highlight its crucial role in maintaining systemic homeostasis. Activation of GPBA has been associated with improved glucose metabolism, enhanced energy expenditure, and reduced inflammation, suggesting significant therapeutic potential in conditions such as type 2 diabetes, obesity, non-alcoholic fatty liver disease (NAFLD), and inflammatory bowel disease (IBD).
Future studies in GPBA receptor biology are essential to further uncover its complex signaling mechanisms and tissue-specific roles. Identification and development of additional ligands — including both agonists and antagonists — will deepen our understanding of GPBA pharmacology and enable the design of targeted therapies with improved efficacy and reduced side effects. Moreover, given GPBA’s expression in the central and peripheral nervous systems, investigating its role in neuroinflammation, neurodegenerative diseases, and visceral pain syndromes presents an exciting frontier. Advances in structural biology, molecular pharmacology, and high-throughput screening will facilitate the discovery of novel modulators of GPBA activity.
In conclusion, elucidating the full biological and physiological functions of GPBA holds tremendous promise for therapeutic intervention across a spectrum of pathophysiological conditions. Continued research into GPBA signaling pathways, ligand specificity, and tissue distribution will likely lead to innovative treatments that address currently unmet medical needs in metabolic, inflammatory, and neurodegenerative diseases.
Conflict of interest: None