Pharmacy and Drug Innovations
OPEN ACCESS | Volume 4 - Issue 1 - 2025
ISSN No: 2994-7022 | Journal DOI: 10.61148/2994-7022/PDI
A.Krishna Sailaja*, G.Vaishnavi,
Department of pharmaceutics, RBVRR Women’s college of pharmacy, affiliated to Osmania University, Hyderabad.
*Corresponding author: A.Krishna Sailaja, Department of pharmaceutics, RBVRR Women’s college of pharmacy, affiliated to Osmania University, Hyderabad.
Received: October 11, 2021
Accepted: October 29, 2021
Published: November 02, 2021
Citation: A.Krishna Sailaja, G.Vaishnavi. “An Overall Review on Formulation and Applications of Lipid Based Drug Delivery System”. J Pharmacy and Drug Innovations, 2(5); DOI: http;//doi.org/03.2020/1.1032.
Copyright: © 2021 A.Krishna Sailaja. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Lipid Based Drug Delivery System (LBDDS) are capable of increasing solubilizing and it results in increasing the bioavailability of the drug by solubilizing the drug in lipid excipients which includes solid lipid nanoparticles, vesicular drug delivery systems. In this review detailed discussion was made upon the various approaches of formulation of lipid based drug delivery system
Introduction
For regulation of drug products world-wide, the biopharmaceutical classification system (BCS) has become an increasingly important tool, since its inception in 1995. Food and Drug Administration (FDA) introduced the classification system , has categorized drugs in terms of their solubility and intestinal permeability. The BCS classification has impact on the worldwide pharmaceutical fields, in discovery of drug, development, and regulation [1,2].
According to BCS classification drugs can be organized into four classes :class I (drugs with high solubility and permeability), class II (drugs with low solubility and high permeability), class III (drugs with high solubility and low permeability) and class IV (drugs with low solubility and permeability) [3,4]. WHO list, 61 out of 130 orally administered drugs classified. 21 (84%) of these belong to class I, 10 (17%) to class II, 24 (39%) to class III and 6 (10%) to class IV.Lipid-based formulations may include oil solution, emulsions, self-micro or self-nano emulsifying drug delivery systems [5,6].
1.1.1 Types of Lipid Based Drug Delivery System
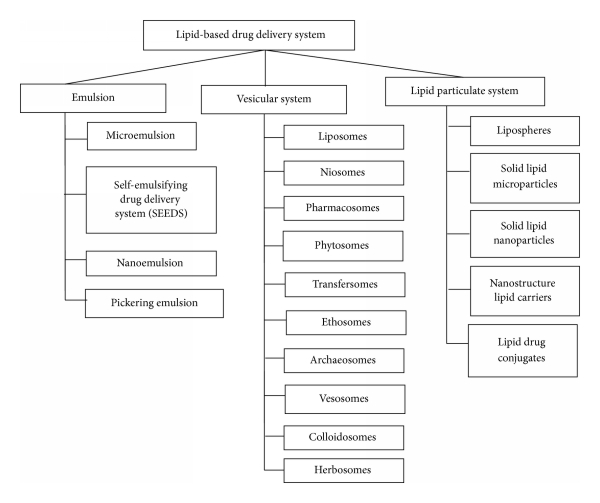
Figure 1.1.1classification of lipid based drug delivery system
1.1.3 Lipid Formulation Classification System
The lipid formulation classification system (LFCS) was found in 2000 as a active model and in 2006 an extra “type” of formulation was added. The LFCs have been discussed more widely within the pharmaceutical industry to lookup a agreement which can be adopted as a framework for comparing the performance of lipid-based formulations In recent years [7,8]. The main purpose of the LFCs is to allow in vivo studies of the formulation can be interpreted easily . With reference to the physicochemical properties of particular drugs, the most suitable formulation can be through detected LFCS. Most of the marketed products are Type III systems. Hence, further divided into Type III A (oils) and Type III B (water-soluble) based on the balance of oils and water- soluble substances [9,10].
|
Type I |
Type II |
Type IIIA (fine emulsion) |
Type IIIB (microemulsion) |
Type IV |
|
Oils without surfactants |
Oils and water insoluble surfactants |
Oils, surfactants, cosolvents (both water-insoluble and water-soluble excipients) |
Oils, surfactants, cosolvents (both water-insoluble and water-soluble excipients) |
Water-soluble surfactants and cosolvents (no oils) |
|
Non-dispersing |
Emulsion (SEDDS) |
SEDDS/SMEDDS formed with water-soluble components |
SEDDS/SMEDDS formed with water-soluble components and low oil content |
Disperses typically to form a micellar solution |
|
Requires digestion |
Will be digested |
Digestion may not be necessary |
Digestion may not be necessary |
Limited digestion |
Delivery Systems Employed in The Permeability Improvement Of Bcs Class Iii Drugs [11,12]
|
Drug |
Delivery system |
Conclusion |
|
Doxorubicin |
Double emulsion system |
In this system drug is available in internal hydrophilic centre which act provide safe environment to drug and act as a storage cavity and from intestine these can get directly absorbed as oil droplets |
|
Acyclovir |
Niosomes |
Niosomal dispersion has improved the oral bioavailability of acyclovir by more than 2- folds as compared to free drug solution because they are osmotically active and chemically stable |
|
Pidotimod |
Self-double emulsifying drug delivery system (SDEDDS) |
In vivo study shows that time profiles of plasma concentration in mice shows increased absorption of pidotimod loaded SDEDDS loaded as compared to its plain solution |
|
Doxorubicin |
Nanoparticles |
Nanoparticles control the release of doxorubicin in a pH dependent manner and enhance the efficacy of drug |
|
Metformin |
Liposomes |
In vivo study performed on metformin-loaded liposomes coated with chitosan cross-linked with beta-glycerophosphate suggested that microcomplexes controlled the delivery of drugs and improve the oral bioavailability |
|
Atenolol |
Solid-lipid nanoparticles |
In vitro permeability study suggested that drug- phosphotidylcholine solid dispersion increases percentage permeation as compared to pure drug and as the amount of phospholipid increases relative to that of drug, permeability of drug increases. This system has ability to enter through various anatomical boundaries, sustained release of their contents and their stability in nanometer size. |
Conclusions
Lipid-based formulations increase the bioavailability of drug by avoiding erratic absorption. The emulsions facilitate the absorption of the drug through intestinal lymphatic pathway and by partitioning of drug into intestinal fluids which is aqueous phase. This technology is a key for formulating low bioavailability drugs.