
Navaira Ali
Liaquat National Hospital and Medical College, Karachi, Pakistan.
*Corresponding Author: Navaira Ali, Liaquat National Hospital and Medical College, Karachi, Pakistan.
Received: November 08, 2022
Accepted: December 02, 2022
Published: January 10, 2023
Citation: Navaira Ali. (2023) “Effect of trastuzumab on disease course in her2neu positive breast cancer in a tertiary care hospital in Pakistan”, J Oncology and Cancer Screening, 5(1); DOI: http;//doi.org/01.2023/1.1064.
Copyright: © 2023 Navaira Ali. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Trastuzumab is a recombinant monoclonal antibody developed to target the Human epidermal growth factor receptor 2 (also known as Her2 neu receptor), it inhibits the proliferation of tumor cells that overexpress Her2 receptors. According to International data and trial results, addition of trastuzumab to chemotherapy for Her2 neu positive breast cancer has led to improvement in prognosis of Her2 positive breast cancer patients. No national data exists of effects of trastuzumab use in Her2 positive breast cancer patients in our patient population, which could tell whether our patients respond to trastuzumab as well or not. Hence, we analyzed the available data in a tertiary care hospital in Karachi where a large number of breast cancer patients are treated.
Methods:
We retrospectively analyzed data of Her2 neu positive breast cancer patients from 2004 till 2017, in Liaquat National Hospital, who had early stage or locally advanced breast cancer and assessed the 2 year and 5 year DFS (Disease Free Survival) and OS (Overall Survival) of patients who received trastuzumab along with chemotherapy and who received chemotherapy alone. As a new Her2 targeted drug was approved and is being used in patients along with Trastuzumab ,thus patients till 2017 who had received single Her2 targeted drugs were included.
Results:
Total 211 records were reviewed and out of them 124 (58.8%) received trastuzumab therapy and remaining were treated with chemotherapy only. The median age of patients was 50 (IQR= 42 – 56) years. The DFS rate at two years between trastuzumab group and the observation group was 83.1% and 72.4% which was not statistically significant (p=0.081).
Five year DFS rate among patients treated with and without trastuzumab was
79.8% and 70.1% respectively without any significant difference (p=0.194). 23 patients succumbed to the illness within two years of diagnosis and 40 succumbed to the illness within 5 years. Two year OS between trastuzumab and the control group was 92.7% and 83.9% respectively (p=0.064). Five year survival among patients receiving trastuzumab was
87.1 % and ones who didn’t receive trastuzumab therapy was 72.4% (p=0.048) and this was found to be statistically significant.
Conclusion:
So our data shows that in our population there was a difference in DFS and OS with the use of trastuzumab along with chemotherapy in an adjuvant/neoadjuvant setting, and it was found statistically significant in terms of 5 year OS of Her2 positive breast cancer patients.
trastuzumab,her 2 neu ; neo/adjuvant chemotherapy; breast cancer
Introduction:
Breast cancer is a disease which is not treated uniformly as it is categorized based on its tissue biomarkers and thus treated differently according to the molecular type of breast cancer [1]. About 70 % of breast cancer is ER (estrogen receptor) positive ,and about 15 to 20 % are Her2 neu positive(HEREAFTER referred as Her2) [2]. Her2 belongs to human epidermal growth factor receptor genes, called ERBB2. The Her2 proto-oncogene is localized to chromosome 17q and encodes a transmembrane tyrosine kinase receptor called p185 Her2 neu. Overexpression of Her2 mediates unregulated cell proliferation, invasion and metastases [3] in cancerous cells thus Her2 positive breast cancer had poor prognosis [4], so much so that patients with metastatic Her2 positive pts had median survival of only 0.7 years [5]. This was because it was said that Her2 overexpression/amplification can lead to lack or lower levels of ER expression, relative resistance to endocrine therapy and higher risk of recurrence [6,7]. But that was until
Trastuzumab, a recombinant humanized monoclonal antibody targeting Her2 receptors was approved by the FDA in 1998. Trastuzumab binds to the extracellular domain of Her2 and prevents cleavage of the extracellular domain of HER-2 and thereby activation of the receptor; blocks the dimerization of HER-2; mediates activation of antibody-dependent cell-mediated cytotoxicity, resulting in tumor cell lysis; and promotes HER-2 internalization(4). It first got approved in metastatic setting as a single agent and showed good clinical response whether used weekly or 3 weekly [8,9] and with chemotherapy as well and showed longer time to disease progression, a higher rate of objective response, a longer duration of response and longer survival [10]. Subsequent phase III trials proved that trastuzumab reduces recurrence rate by about 50 % in early breast cancer patients [11]. There are 5 randomized trials regarding Trastuzumab that demonstrate the addition of Trastuzumab to chemotherapy as well as
adjuvant treatment for Her 2 neu-overexpressing breast cancer. The HERA trial, a large randomized phase III trial examined both, 24-month and 12-month, durations of trastuzumab treatment compared with a no trastuzumab control group and reported a benefit of DFS at 2 years of 8.4% (95% CI 2·1–14·8) for the 12-month treatment group compared with the no
trastuzumab group [12]. The joint analysis of the US studies (NSABP-B31 and NCCTG-N9831) showed a similar hazard ratio (HR) for 12-month trastuzumab and a control group that did not receive this drug, with a benefit of disease-free survival of 11·8% at 3 years [13]. In 2006 a
smaller study, FinHer [14] investigated giving 9-weeks of trastuzumab with chemotherapy versus chemotherapy alone; it showed very similar results with a benefit in disease-free survival of 11.7% at 3 years for 9-week trastuzumab. This result prompted a substantial interest in curtailing Trastuzumab to 9 weeks as this might be cost effective in developing countries where patients have financial constraints. But as it was a small trial thus its results are not validated.
All the adjuvant trials incorporated chemotherapy alongwith trastuzumab, there is no data to confirm whether trastuzumab alone without chemotherapy can be effective. The optimal chemotherapy backbone for trastuzumab-based adjuvant treatment is uncertain [15]. Most patients treated on the clinical trials received sequential anthracyclines and taxane-based treatment, with concurrent use of trastuzumab during taxane treatment. The results from BCIRG 006 suggest that the nonanthracycline trastuzumab, docetaxel, and carboplatin (TCH) regimen is superior to chemotherapy given without trastuzumab. TCH is an important treatment option, particularly in patients with contraindications to anthracycline-based treatment, corroborated by the 10-year follow-up of the BCIRG 006 study that showed equivalent DFS and OS in the two trastuzumab-containing arms but with lower risk of cardiac adverse events and leukemia in the
non-anthracycline TCH arm [16]. Trastuzumab is active when delivered sequentially after chemotherapy, as done in the HERA trial [12], or concurrently with chemotherapy, as done in the NSABP B-31/North Central Cancer Treatment Group [NCCTG]N9831[13] and Breast
Cancer International Research Group [BCIRG] trials [17]. However, it is seen that concurrent Trastuzumab yields superior results as compared to sequential therapy [18]. So our aim was to primarily compare the results of 1 year of Trastuzumab with no Trastuzumab use in the population under treatment for Her2 positive breast cancer at our tertiary care hospital.
Methods:
We retrospectively analyzed data from our patients files in the Medical Oncology department, Liaquat National Hospital which is a tertiary care center of Karachi, Pakistan. Data collected from year 2004 upto year 2017 was included. The study approval was taken by the institution's ethical review committee. It was not funded by any sponsor.
Patients were eligible if they were of age 18 years or older, diagnosed with primary invasive early or locally advanced breast cancer, and were found to have Her 2 neu overexpressed or amplified breast cancer [19]. A result on immunohistochemical analysis (IHC) of 3+ in a range from 0 to 3+ with higher values indicating increased overexpression, was required for
confirmation of the status of tumors and/or a positive result on fluorescence in situ hybridization (FISH) for HER2 amplification was required for tumors that were assessed IHC 2+ .
All patients had clear indications for treatment and provided written informed consent. Patients were excluded if they had Her2 negative disease, or had metastatic breast cancer or any other concurrent malignancy along with breast cancer.
Patients received chemotherapy (neo-)adjuvant as per the requirements according to their stage of disease, which consisted of taxanes and/or anthracyclines. For patients receiving trastuzumab with taxanes, if taxanes were administered in a weekly manner then trastuzumab was given at a loading dose of 4mg/kg and subsequently 2mg/kg and then on completion of chemotherapy the trastuzumab was switched to being administered 3-weekly at 6mg/kg. If taxanes were given in 3-weekly doses, trastuzumab was administered at an 8mg/kg loading dose followed by 6mg/kg every 3 weeks. Standard was to give it for 12 months in total which was about 17 cycles. Patients underwent definitive surgery and/or radiotherapy, if applicable. Patients who were hormone positive received hormonal therapy along with trastuzumab after completion of their chemotherapy. Mostly premenopausal patients were given Tamoxifen and postmenopausal patients were given Aromatase Inhibitors.
Patients were categorized into two groups, one who received trastuzumab along with chemotherapy and the other was the one who did not receive trastuzumab and received chemotherapy alone. Table 1 lists baseline characteristics of the patients, tumor stage and primary treatment. The baseline characteristics of the two groups were well balanced.
Outcomes:
Primary endpoint was 2-year and 5-year overall survival and it included death from any cause, and was calculated from the completion of chemotherapy to the event when it occurred.
Secondary endpoint was 2-year and 5-year Disease free survival (DFS) with DFS events including loco-regional or distant recurrence of breast cancer, development of contralateral breast cancer and was calculated from the end of chemotherapy completion to the event when it occurred.
Results:
Study Population:
Total 211 records for breast cancer patients were reviewed. The age range for patients is 25-80 years with median (IQR) age of 50 (42 – 56) years. Most of the patients presented with stage 3 tumor (n=130, 61.6%) while remaining presented with stage 2 (n=75, 35.5%) and stage 1 (n=6,
2.8%)(TABLE 1) Majority of the study participants had tumor size of T2 (82, 38.9%) and T3 (n=85, 40.3%) whereas few also presented with T1 (10, 4.7%) and T4 (34, 16.1%). 75(35.5%) patients had involvement of 1 to 3 lymph nodes while nearly quarter had no involvement (n=56, 26.5%) and
less than 20% presented with 4 to 9 lymph nodes(n=42, 19.9%) or more than 10 lymph nodes (35, 16.6%) involvement. Estrogen and progesterone receptor were positive among more than half of the participants (n=111, 52.6%). Almost half of the patients treated with neoadjuvant
(105, 49.8%) and other half with adjuvant therapy (n=105, 49.8%) and only single patient didn’t receive chemotherapy. Majority of the patients received radiation therapy (n=167, 79.1%) either because of breast conservation surgery being done or because of size of tumor being more than 5 cm or lymph node positive disease. Nearly all of patients were managed with surgery (n=206, 97.6%).About half of the study participants didn’t require hormonal therapy (n=104, 49.3%) because of hormone receptor negative disease and remaining received tamoxifen (n=62, 29.4%) and aromatase inhibitors (n=45, 21.3%). More than half of the patients received trastuzumab therapy for a year (n=124, 58.8%)(TABLE1)
Efficacy:
A total of 27 patients experienced two year disease recurrence event and out of them, 13(10.4%) belonged to the trastuzumab group and 14(16.0%) belonged to the chemotherapy only group (Table 2). Within 5 years, disease recurrence events were observed in 45 patients. 22(17.7%) belonged to trastuzumab group and 24(26.4%) to chemotherapy only group.
Two year disease free survival rate among patients receiving trastuzumab therapy was higher than those who didn’t receive it (83.1% vs. 72.4%) but without any statistical significance (p=0.081)(TABLE 3).
There was no statistical significant difference between the two different groups for 5 year DFS (79.8% vs. 70.1%, p=0.194). 23 patients expired within two years after starting their treatment. 92.7% patients survived in
trastuzumab and 83.9% in the chemotherapy only group. The insignificant difference was observed among the two study groups (p=0.064). There were a total of 40 mortalities within five years, out of which 16 mortalities were in the trastuzumab group while 24 mortalities in chemotherapy alone group. Five year overall survival for trastuzumab and control group was
87.1% and 72.4% respectively. There was a statistically significant difference seen in 5 year
Overall Survival(OS) rates between both the studied groups(p= 0.048)which proved that our population shows the benefit of using Trastuzumab along with chemotherapy in Her2 positive breast cancer patients(TABLE 3)
Table 1: Baseline characteristics of the patients, tumors and primary treatments (intention to treat groups)
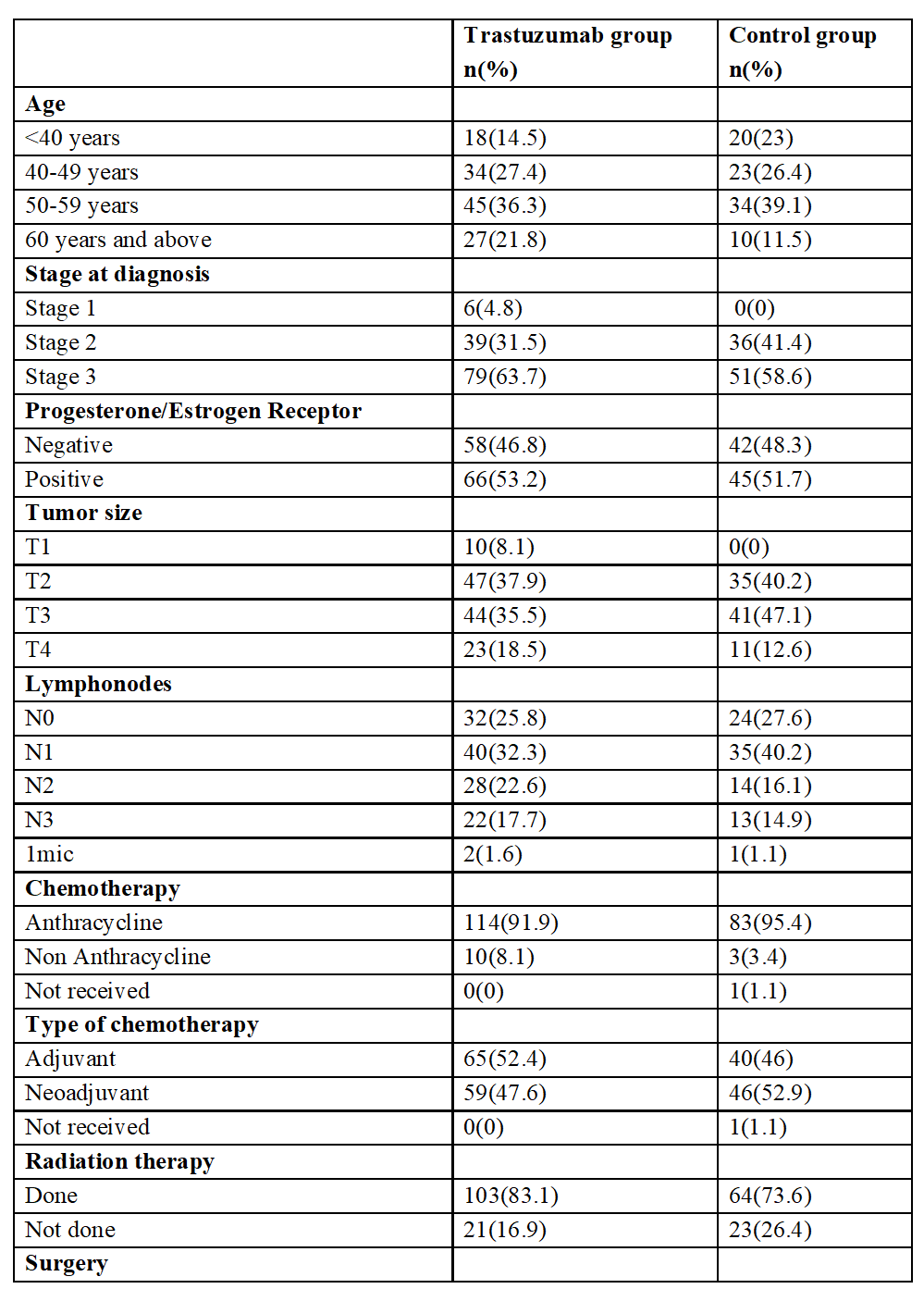
Table 2: Details of clinical events
Table 3: Survival rates among trastuzumab group and control group Log Rank Test is applied.
*Significant at p≤0.05

Table 4: Two and five years disease free and overall survival rate in different factors with respect to treatment (with p-value)

Chi-square test is appliedƚ Fisher-Exact test is reported
*Significant at P<0.05
Only subgroups that had statistically significant p value have been included in this table.

Figure 1: Two-year disease free survival among patients received trastuzumab for a year and control group

Figure 2: Five-year disease free survival among patients received and did not receive trastuzumab therapy
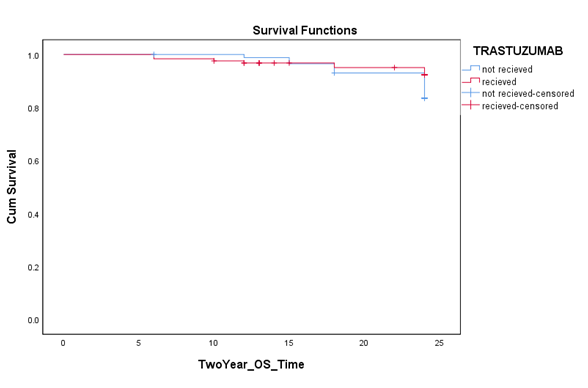
Figure 3: Two year overall survival among patients treated with and without trastuzumab therapy
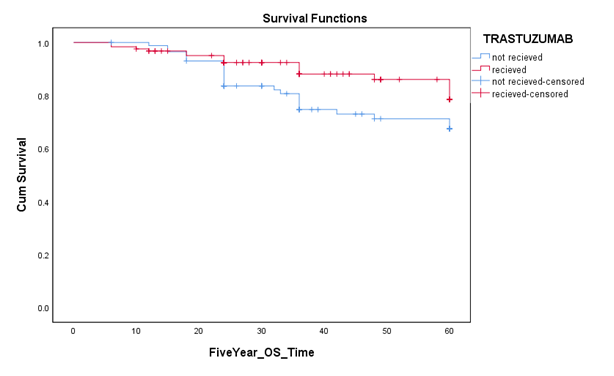
Figure 4: Five year overall survival among patients who received and didn’t receive trastuzumab therapy
Discussion:
In the past, Her 2 positive breast cancer connoted aggressive disease with increased risk of recurrence and death. Understanding the molecular pathways(10) tied to Her2 receptors led to generating targeted agents like trastuzumab which has improved prognosis of Her2 positive breast disease. As trastuzumab showed its efficacy in metastatic carcinoma of the breast, it also proved its mettle in adjuvant settings, in all randomized trials. Due to limited resources, it was quite difficult to incorporate Trastuzumab in our practice in developing countries. Thus, very limited data is available regarding its use in our population. We retrospectively analyzed data of our patients and compared the difference in populations treated with trastuzumab and the ones who did not receive it.
We kept a minimum time of 2 years for follow up for our analysis thus took patients till 2017, but as we had included patients from year 2004 thus there were some patients with 13 years follow up too. According to our data analysis, the use of trastuzumab for 1 year did improve DFS in our population, when administered in addition to standard of care, including chemotherapy in patients with early breast cancer who are Her2 positive .There was an absolute benefit of 9% in DFS in women who received trastuzumab compared with those who did not receive it. On the other hand, 2 year OS was equivalent and there was not much of a difference, which may be because it was too early to assess survival benefit over a short span of time. Then we also assessed 5 year DFS and OS of the same population, which showed survival benefit of 14.7 percentage points and it had a p-value=0.048 and was statistically significant among patients receiving trastuzumab. Thus, our query regarding the effect of trastuzumab on our population was answered and our study shows that trastuzumab does benefit patients with HER2-positive breast cancer.
Interestingly, there was no difference in DFS and OS for patients who received trastuzumab with chemotherapy or chemotherapy alone in adjuvant setting (Table 4) Conversely, in patients who had received neoadjuvant chemotherapy and trastuzumab, they had 13.9% improvement in 2 year DFS and 17 % improvement in 5 yr DFS . There was slight difference in 2 year OS but it was not statistically significant and later ON ,on 5yr OS analysis, it was found that use of trastuzumab translated into statistically significant difference.
In our population, patients with hormone negative breast disease had lower DFS and OS in comparison to the hormone positive population. This is similar to the results of Hera trial [23]. This means that Hormone negative, Her2 positive disease are more aggressive than triple positive disease.
Patients had difference in their DFS and OS according to Tumor size . It was found that tumors which were more than 5 cm or had skin involvement, showed marked difference in their survivals with use of trastuzumab.(Table 4) This shows the need of neoadjuvant therapy of her 2 positive disease ,as it is aggressive and thus giving systemic therapy for tumors that are node positive and more than 2 cm have become a standard [21,22].
In our study during 5 years, 8 patients who received trastuzumab developed brain metastases whereas only 2 patients in non-trastuzumab developed brain metastases. This also reinforces that large molecules such as chemotherapeutics and monoclonal antibodies have decreased concentration of drugs in the brain due to blood brain barrier, as compared with extracranial lesions [25]. Response rate of conventional cytotoxics is around 50% in the brain. Once the blood brain barrier is disrupted secondary to brain metastases then these agents can penetrate and exert their effect. Recent trials also show that controlling systemic disease prolongs brain metastases free survival but does not reduce the overall incidence of brain metastases [24].
As we did not have a large sample size thus we included both the patients who received neoadjuvant therapy and adjuvant therapy in our study, it would have been better to present the data in two separate studies. We could have given results of DFS and OS according to pathological response as complete pathological response is taken as a surrogate marker for imorved survival but as we did not keep it as our objective thus it was not inferred.
Conclusion:
Trastuzumab use in our population translated into improvement in overall survival and thus targeting Her2 receptors remains the cornerstone of treatment in Her2 positive breast cancer patients.