Neurosurgery and Neurology Research
OPEN ACCESS | Volume 7 - Issue 1 - 2025
ISSN No: 2836-2829 | Journal DOI: 10.61148/2836-2829/NNR
Tshetiz Dahal
General Physician - Lugansk State Medical University, Luhansk Oblast, 93000 Luhansk, Ukraine
*Corresponding Author: Tshetiz Dahal, General Physician - Lugansk State Medical University, Luhansk Oblast, 93000 Luhansk, Ukraine.
Received date : March 16, 2022
Accepted date : April 21, 2022
published date : May 06, 2022
Citation: Dahal T, (2022) “Pyogenic - Cerebral (Brain) Abscess”. J Neurosurgery and Neurology Research, 4(2); DOI: http;//doi.org/011.2022/1.1041.
Copyright: © 2022 Tshetiz Dahal. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abscesses in the brain have been one of the most challenging wounds, both for surgeons and trainees. It is a packet full of pus of infected material in the part of the brain. It is an important neurological disease and can produce deadly diseases. Since the beginning of the era of computed tomography (CT), the diagnosis and treatment of these organizations has become simpler and less invasive. The results have been improved with the development of diagnostic techniques, neurosurgery, and comprehensive antibiotics. Rare bacterial abscesses are usually caused by the use of chemicals in oncology, longevity in patients infected with human immunodeficiency virus (HIV), and immunosuppression associated with organ transplants. Surgical treatment options did not show significant differences in mortality rates, but lower rates of illness were achieved with a stereo-guided curiosity. Eager stereo-directed antidepressant, antibiotic-based treatment based on the effects of culture culture, and repeated desires expressed in the results of periodic CT scans appear to be the most appropriate treatment for brain tumors. Immunosuppression and comorbidities, early neurological conditions, and intraventricular fractures were important factors influencing patient outcomes. Traps and mutations in the diagnosis and treatment of brain tumors have been discussed in this study.
Introduction
In 1893, Surgeons reported only 1 death in 19 patients with brain tumors. Unfortunately, until the advent of the CT method, the results in patients with brain tumors were not as satisfactory as in the Macewen series.
The use of CT and MR imaging, the emergence of microbiological diagnostic techniques, and the production of comprehensive antibiotics have improved the results over the past 20-25 years. Regular use of CT scans has facilitated the detection of tumors in the brain and makes patient follow-up safe. The mortality rate drops from 22.7-45% to 0-20% after regular use of CT scans. Prior to the advent of CT scans, brain tumors were more commonly diagnosed and removed completely. However, simpler and more secure diagnostic techniques make stereotactic aspiration a viable treatment option, especially for large and non-cortical ulcers. Also, in some cases CT scan makes safe and effective treatment without surgical intervention. However, there is no consensus on the treatment of tumor in the brain; the need for surgical intervention and the nature of the surgical procedure is still in doubt.
Abbreviations used in this paper
ADC = diffusion coefficient, CHD = congenital heart disease, CNS = central nervous system,
CSF = cerebrospinal fluid, DW = diffusion weighted
The Origin of Abscesses
Cerebral abscess occurs in patients with the following predominant conditions:
1) Conjunctival pus (for example, anterior sinus infection leading to the anterior cruciate ligament, sphenoid sinus infection leading to enlargement of the cavernous sinus, and an infection of the middle ear / mastoid air cell leading to the temporal lobe and spinal cord).
2) Hematogenous or metastatic spread (e.g., lung and arteriovenous shunts, congenital heart disease and endocarditis, dental diseases, and gastrointestinal diseases).
3) Headaches.
4) Neurosurgical Procedure. And
5) Immunosuppression.
According to previous literature, the most common cause of boils in the brain was direct transmission from the middle ear, meninges, mastoid infections and the paranasal sinus. Prior to the 1980s, CHD (6–50%) and sinus / otitis infections appeared to be the most common symptoms in brain tumors and in children. The advent of diagnostic techniques, antimicrobial agents, and advances in cardiovascular surgery have led to a decrease in the number of tumors in the brain due to CHD and sinus / otitis infections and an increase in ulcers in patients receiving immuno suppressive therapy as a result of reconstructive procedures, in patients. HIV-positive people who have been living longer, and those who are receiving chemotherapy for cancer treatment. Many abscesses appeared in the 1980's in infants and patients with depressive disorders, and they were diagnosed early, (6 months).
Nowadays, hematogenous or metastatic proliferation has become a very common factor in brain tumor formation. Organisms that cause tumors in the brain are usually derived from bacteria. Pepto-streptococcus and Streptococcus spp (especially S. viridans and microaerophilic organisms) are more common in patients with coronary heart disease (cyanotic heart disease) and shunt bypasses from right to left without the usual filtration of pulmonary vascular tissue. . In CHD, decreased arterial oxygen uptake and increased blood viscosity may cause focal cerebral ischemia and serve as a nidus for many diseases, especially in the combination of gray matter and white matter, usually in the spread of MCA. CHD has sometimes been an important cause of childhood ulcers, but there has been a decline in these cases due to advances in heart surgery and the use of broad-spectrum anti antiretroviral drugs.
Bactericidal, Pepto streptococcus, and Streptococcus spp are often identified in brain tumors caused by a combined spread. This spread is the result of osteo myelitisin the surrounding air sinus. The risk of developing cerebral palsy in an adult with active otitis media is ~ 1 / 10,000 per year, but in a 30-year-old patient with active infection, the life-threatening risk is ~ 1/200.
Streptococcus, S. aureus, Pseudomonas, and Bacteroides spp are more common in lung diseases (lung abscess, empyema, bronchiectasis). They are most common in the distribution of MCAs and are often repetitive.
Staphylococcus, Streptococcus, Clostridium, and Enterobacter spp are more common in patients with open-headed trauma. Shoot wounds, open skull openings and external bodies in the brain parenchyma, and fractures of the skull with CSF fistula cause abscesses in the brain, often associated with traumatic brain injury.
Staphylococcus and Streptococcus spp are identified as inpatients who have previous neurosurgery procedures. Wounds in the opening hours are less likely to be infected. Additional risk factors include implantation of an external body such as a shunt or external ventricular drain, advanced gliomas, and premature radiation exposure after surgical procedures.
Fungal Infections
Toxoplasma, Staphylococcus, Streptococcus, and Pseudomonas spp are identified in non-immune patients who are infected with HIV, organ transplants, chemotherapy, or steroid use. Branch-infected fungal infections (e.g., aspergillosis) block large and medium-sized vessels, resulting in cerebral arterial thrombosis and infarction. Sterile infarcts can be converted into septic infarcts by a tumor-related structure. Abscesses can also be caused by a joint spread.These lesions are most commonly found in the posterior fossa and lobe of the brain. The mortality rate due to fungal tumors ranges from 75 to 100%, despite strong treatment with amphotericin.
It still has a strong representation of anaerobes (30-50%) in patients with brain tumors. Additionally, atypical bacteria such as Nocardia and Actinomyces spp may appear in affected patients. Although positive civilization rates have reached 100% in studies of careful management of clinical models, adverse cultural events remain as high as 15-30% in most series, especially in patients where antimicrobial treatment is initiated before surgery.
Polymerase chain reaction analysis of S recombinant DNA and sequence may identify viruses at the level of species from tumors in the brain. This method is fast and useful especially in identifying slow-growing and fast-moving organisms.
Lumbar puncture has been considered dangerous for patients with a brain tumor. It is usually caused by strong suspicion of concomitant meningitis and / or ventriculitis, and reveals only 10-30% of positive CSF cultures in which organisms such as those implanted in the gut are found. Although a large portion of the deaths were thought to be caused by lumbar piercing during the onset of labor, recent studies in which multivariate modifications were used have failed to reveal such a risk. Therefore, lumbar piercing may be recommended in patients with brain tumors where there is no increase in intracranial pressure and with clear signs of meningitis and / or ventriculitis.
Pathogenesis of Brain Abscesses
Abscesses in the brain develop as a result of parenchymal infection by pyogenic bacteria, starting as a local cerebritis and transforming into a strong wound surrounded by a well-vascularized fibrotic capsule. Brain tumors in humans have been based on diagnostic findings during CT scans or MR imaging sessions.
The first stage or early cerebritis occurs from Day 1 to 3 and is characterized by the accumulation of neutrophil, tissue necrosis, edema. Microglial and astrocyte activity is seen in this phase and continues throughout the development of the tumor.The intermediate phase, or late cerebritis, occurs in Days 4 to 9 and is associated with macrophage infiltration and lymphocyte.
The final or capsule stage occurs from Day 10 onwards and is associated with the formation of a well-developed vascular wall, which actually closes the wound and protects the normal brain parenchyma from further damage. The capsule formation is usually early from 10 to 13 days and is usually thin on the medial or ventricular side of the abscess and usually ruptures in this way.
After Day 14, late capsule formation develops, with gliotic, collagenous, and granulation layers.12 In addition to reducing the rate of infection, the immune response that is an important part of tumor formation also destroys surrounding normal brain tissue. This support is found in experimental models, in which harmful areas are exaggerated in comparison to the natural environment for bacterial growth, which is reminiscent of an overactive immune response. of brain tissue, which often spreads beyond the initial focus of infection. Therefore, controlling the intensity and / or duration of the antibacterial immune response in the brain may allow for effective elimination of bacteria while minimizing damage to the surrounding brain tissue.
As mentioned earlier, injury sites in both experimental models and tumors of the human brain are exaggerated in comparison to the natural environment of viral growth, the eminiscent of the most effective immune response. Reporting on the extended region of affected tissue involvement associated with brain tumors compared with the condition focused on initial shock, Kielian suggested that the production of inflammatory mediators following S.aureus infection continue, effectively increasing damage to the normal surrounding brain parenchyma. Specifically, the continuous release of pro-inflammatory mediators by activated glia and internal immune cells may work through a constructive response loop to enhance the recruitment and utilization of newly recruited inflammatory cells and glia. This will effectively promote the antibacterial inflammatory response through a vicious pathological circle culminating in extensive collateral damage to normal brain tissue.
Recent research supports the ongoing immune function associated with experimental tumors, in which high levels of interleukin-1b, tumor necrosis factor – a, and macrophage inflammatory protein – 2 were detected between 14 and 21 days after exposure to S.aureus. In line with the long-term inflammatory mediator, TS aureus infection was found to cause chronic disruption of the blood-brain barrier, which is associated with the continued presence of peripheral immune cells and glial function. Overall, these findings suggest that interventions with anti-inflammatory compounds following adequate bacterial neutrality may be an effective strategy to reduce damage to the surrounding cerebral parenchyma during brain tumor development, leading to improved cognitive and sensory effects.R microglia and microcyte-microglia responses in S. aureus is defined in terms of the burning mediator's expression, and is generally found to be of the same quality as those detected after lipopolysaccharide exposure. Although studies containing basic microglia and astrocytes from Toll-like receptor knockout mice reveal an important role for this receptor in regulating S-dependent activity. aureus, it is clear that additional receptors are also involved in the inglial response to this virus. is surprising because these pathogens have the potential to have harmful effects on tissues such as the CNS, which have limited ability to regenerate.
The effects of glial cell function on brain tumor context are almost numerous.
01. First, parenchymal microglia and astrocytes may be involved in the initial recruitment of specialized bactericidal phagocytes in the CNS by their expression of inflammatory chemokines and cytokines.
02. Second, microglia exhibit bactericidal activity of S. aureus in vitro, suggesting that they may also be involved in the initial inhibition of viral replication in the CNS. However, their bactericidal in vitro activity does not compare with that of neutrophils or macrophages, suggesting that this activity may not be a major mechanism of microglia effect of severe infection.
03. Third, activated microglia have the potential to influence the type and level of antibacterial adaptive immune reaction through its phase II complex administration of the histo-compatibility complex and exposure to the costimulatory molecule. Finally, if glial activity persists in the context of progressive inflammation, continuous release of inflammatory mediators can damage the normal brain parenchyma.
Clinical Presentation
There are no pathogenic clinical symptoms; Most patients with clinical symptoms depending on the location or major impact of the lesion: headache, nausea, urination, fever, dizziness, fainting, and muscle spasms are the most common symptoms. These symptoms develop rapidly, however. about tumor lesions. The flu is less pronounced, and only 30-55% of patients with the flu are 38.5ºC. Capture is a symptomatic mark in 16-50% of patients.Focused neurological loss is seen in 40-60% of patients, depending on the location of the lesion. Papilledema is not available to patients, who are 2 years old. Patent sutures with low ability to limit infection and cranial enlargement are possible. However, three symptoms of brain tumor (headache, fever, and neurological deficits) can only be seen in 15-30% of patients. paralysis, muscle weakness, and many different symptoms may be present and degeneration is often very rapid.
Diagnosis
The photographic features of the tumor in the brain depend on the stage at the time of photography and the source of the infection.
The development of an abscess in the brain can be divided into 4 stages:
1) early cerebritis (1-4 days); 2) late cerebritis (4-10 days); 3) formation of the first capsule (11-14 days); and 4) capsule formation late (14 days).
Most abscesses show a lot of surrounding edema, which usually occurs during late cerebritis or the first stage of capsule formation, which follows a major effect. Blood clots, which can be seen in the form of endocarditis, cardiac shunts, or paralysis of the pulmonary vascular malformations, usually numerous, are identified at the junction of gray and white, and are found in the MCA area. In the earlier stages, a CT scan was performed without the addition of brightness can only show low comfort with great effect. In the later stages, a complete peripheral ring may appear. For CT scans obtained after the administration of different materials, the development of the same ring remains in the later stages. In the early stages the capsule will be difficult to visualize using conventional techniques, and double-sided CT therapy is often helpful in interpreting tumor closure. Metastatic tumors, advanced gliomas, cerebral infarction, cerebral palsy or hematoma, lymphoma, toxoplasmosis, debilitating disease, and radiation necrosis should be remembered as a separate diagnosis of brain tumors that appear as enlarging lesions ring.
techniques in neuro radiology have simplified the diagnosis of multiple brain tumors. The incidence of multiple abscesses in the brain, which is reported to be 1.8-17% of patients
the pre-CT period, 23–50% in modern cases. The findings of MR imaging also depend on the stage of infection. In the first stage, lesions exposed to MR images may have a low T1-weighted signal and a high signal in T2-weighted, enhanced and scarred images. , with a high signal in T2-weighted images, both in the pit and surrounding parenchyma.The abscess shows hyper-intense rim in T1-weighted images obtained indistinctly and a strong rim in T2.As-based images in CT scanning, imaging MRI usually show a developmental ring around the abscess.The abscesses usually grow to a white matter, away from the gray-vascularized gray matter, as well as narrowing of the middle wall. However, the mark of the developing ring is not specified and should be examined in the context of clinical history. The size, inconsistency, and stiffness of the developing ring elevate the tumor (most cases), or possible fungal infection (Fig. 1).
In DW images, limited streaming (bright signal) may be visible; this helps to separate tumors from necrotic neoplasms, which are usually not prevented, although not all tumors follow this rule. Fungal abscesses and tuberculosis may increase the distribution and low signal in DW thinking.
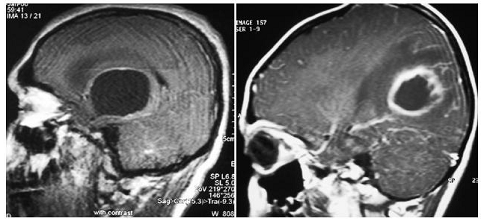
Figure 1: MR images with Sagittal T1 weight obtained in different patients after different treatment, showing cystic lesions that improve the ring.
- Left: MR admission image reveals a small, straight, well-marked cystic lesion with central perilesional edema. Pathological examination revealed cystic pilocytic astrocytoma.
- Right: MR reception image showing thick, distinct (cortical tension, thin on the ventricular side), peripheral lesion with perilesional inflammation and constipation due to vasculitis and cerebritis of the surrounding parenchyma.
Pathological and microbiological examination reveals a pyogenic brain tumor.
Numerous studies show the benefits of DW imaging in distinguishing between necrotic or cystic ulcers and brain tumors. ADC. Initially, DW hypotheses were thought to be helpful in differentiating toxoplasmosis and lymphoma.In a study ADC limit of 0.8 was proposed, where ADC values, 0.8 may favor lymphoma over toxoplasmosis; However, that study showed a significant increase in ADC values in toxoplasmosis and lymphoma. As the author concluded that in most patients, ADC values are not clear in making a distinction between toxoplasmosis and lymphoma. However, DW imaging has a high sensitivity to the detection of rapid ischemic changes in the cortical and deep white matter that can occur in cases of infectious vasculitis. The tumor hole in the brain shows regions of increased levels of partial anisotropy, which have a relative variability compared to other cystic intracranial lesions. This information may prevent misinterpretation of diffusion tensor image information as abnormal mass of white matter fiber associated with large lesions. Intracerebral tumors are characterized by certain resonances in MR spectroscopy that are not detected in a sterile human tissue. MR spectroscopy modality has been shown to be particularly useful in distinguishing between brain tumors and other cystic ulcers, information that can be used to speed up the implementation of appropriate antimicrobial treatment. Metabolic substances, such as succinate (2.4ppm), acetate (1.9 ppm), alanine (1.5 ppm), amino acids (0.9 ppm), and lactate (1.3 ppm), may all be present. in bacterial tumors that can be treated or immediately after initiation for treatment.
Treatment
There are 3 treatment options for brain tumors:
1) Medical
2) Respiration (freehand, stereotactically or neuro endoscopically guided).
3) Total cut
In choosing the appropriate treatment option, the following factors should be considered: Karnofsky performance measure; primary infection; predisposing condition; and number, size, location, and stage of the tumor. Modern treatments for brain tumors often include a combined surgical and therapeutic approach.
Medical Management
Antibiotics play a key role in regulating abscesses in the brain. The agent's characteristics (such as brain infiltration) and previous use of intrathecal or interstitial treatment should be known prior to treatment. Choosing the right antibiotic, microorganism or underlying disease should be identified. If the patient is not in sepsis or in a critical situation, antibiotic treatment should be postponed until cultural factors are identified. We have reported eight times the number of invasive cultures in patients receiving antibiotics before surgery. The author reported that intra-cerebral material cultures remain infertile (34%) of their surgical patients. Of the 76 patients whose culture was infected, (89%) identified one virus and (11%) viruses were detected. If a predisposing condition is spread with hematogenous or patients have symptoms of systemic infection, blood tests may be helpful in identifying the microorganism. After performing blood tests on 49 of the 122 patients who had a clinical presentation of systemic infection (fever and leukocytosis). Only 13 of those patients had blood clots that raised viruses (positive rate, 26.5%); Seven of them had the same pathogen in both cultures of the blood and brain tumors. Blood test is a very small, inexpensive, and quick way to detect a pathogenic microorganism. Despite the low levels of positive findings, blood tests should be taken from every patient who is suspected of having a brain tumor and who has symptoms of systemic infection. Only medical treatment can be considered if patients are poor partners of surgical intervention in the following terms: if the lesions are numerous; 1.5 cm wide; found in clever places; or if there are concomitant diseases such as meningitis or ependymitis. The most important objection to empirical treatment without microbiological identification; another microorganism may cause an abscess. At least one aspiration procedure can be very helpful in identifying a microorganism, if the patient does not have coagulopathy. Treatment alone is most effective if treatment is started during cerebritis, if the wound is 1.5 cm wide, if the duration of symptoms is 2 weeks, and if the patient shows improvement in the first week. Systemic antibiotics have been given for 6 weeks, although some institutions now offer 2 weeks of intravenous antibiotics followed by up to 4 weeks of oral antimicrobial treatment. If the microorganism is undetectable, 6-8 weeks of intensive treatment may be required.
Despite appropriate treatment, a 5-10% recurrence rate has been reported in brain tumors, which can be caused by discontinuation of treatment early. I reported a series in which the duration of antibiotic treatment was not based on a specific time but rather on the levels of C-reactive protein levels. In addition, high levels of C-reactive protein can be used in differentiating the tumor of the brain from other lesions that improve the ring. Three of the 26 patients had been elevated C-reactive protein levels and were found to have recurrent abscesses. It did not happen again in patients their levels returned to normal. Antibiotics and hyperbaric oxygen therapy were given for a total of 4 weeks in 13 patients, even in patients who did not have a bacteriological diagnosis. Overall, the first operation failed in 2 patients (15.3%). Two re-emerging tumors were resuscitated for 6 and 9 days, respectively, after the initial procedure. However, long-term radiological tests failed to show recurrence of tumors in any of these conditions after a 9.5-month follow-up period. The main difference between their research and other reported cases in the reduced duration of antibiotic treatment.
Nowadays, with easy radiological monitoring of brain tumors and extensive antibiotics, doctors often choose treatment, especially if the pathogen can be detected based on blood culture, CSF, or specific desire. It was reported in 56 patients who were randomly selected for treatment, anticipation, or surgery for their brain tumor and found no statistically significant difference. In fact, boils in the brain cause significant physical stress in patients, and surgical stress should not be added if necessary. Corticosteroids can be used, but they have side effects, and their use in the treatment of vasogenic edema due to an abscess on the brain is still debated. The adverse effect of dexamethasone on capsule formation has been demonstrated in experimental studies and made similar comments about the effect of corticosteroids. However, it is reported that corticosteroids do not inhibit capsule formation, and that they only act as reversible forces. Mampalam and Rosenblum reported high mortality rates in patients treated with corticosteroids, but these patients were in a critical state at first and had decreased levels of awareness. These authors recommend the use of corticosteroid in patients with significant perilesional edema that was radiologically diagnosed and reported in a previous study of 26 patients who showed no adverse effect on the effect when corticosteroids were used in patients with intracranial tumors. It should be borne in mind that steroids may reduce the brightness of the tumor capsule in the early stages of infection and that this may be a false indication of radiological development, or it may delay diagnosis.
Surgical Management
Throughout the history of neurosurgery, treatment of brain tumors has been challenging. Non-surgical treatment of suspected small brain tumors with antibiotics has been recommended. Reasonable control of intracranial mass lesions requires the establishment of a thorough diagnostic prior to the initiation of therapeutic measures. Indeed, patients presenting with progressive progressive neurological deficits resulting in a significant neuro-radiologically verified brain tumor by neuro radiologically verified decompression, both by surgeons and internists. Different types of surgical procedures have been used in the treatment of brain tumors. .
The choice of procedure has been the subject of much debate. Craniotomy, previously promoted in the past when no antibiotics or CT scanning was available, is no longer used. Breathing, repeated as needed or by draining water, has replaced the exhaustion efforts completely.
However, an open surgery procedure is still preferred to treat brain tumor with a combination of treatment and surgical removal, in the following cases: if there is evidence of increased intracranial pressure due to a major brain tumor effect; there is difficulty in diagnosis; if the abscess is the result of traumatic injury presented by foreign objects; if the wound is located in the posterior fossa; and if there is any consideration of fungal infection. Even stress with Craniotomy or Craniectomy will be helpful for patients with a serious emotional condition.
Because diagnoses based solely on clinical radiological and neuro-radiological findings may be erroneous, non-surgical treatment decisions should not be made without a thorough pathogen diagnosis. Control of the brain stereo strategy in the brain, which allows for both diagnostic and therapeutic confirmation of the desired wound content and identification of the traumatic organism, has expanded since the introduction of CT-guided stereotaxy. A recent review of the literature shows a series of tumors in the brain treated primarily with stereotactic techniques and a review of their 11-patient series, concluding that stereotactic cravings should be considered as an alternative treatment for all but the most external and major and related tumors. failure of stereotactic treatment of tumors in a series of 29 cases, due to insufficient appetite, lack of catheter drainage, prolonged immunosuppression, or lack of antibiotic treatment.
It was therefore reported that 4 patients with brain tumors underwent surgery using a free-neuro endoscopic method with freehand stereotaxy. They breathe in pus and clean the hole with antibiotics. The author reported their self-awareness with a flexible scooter (free-directed or natural-directed), while choosing the strongest in the children’s series. It has now reported the usefulness of flexible endoscopes for certain important surgical procedures, such as wishing and examining the tumor in all local directions or dealing with a strong and elastic membrane that requires scissors or other implants. It is now maintained that drainage catheters do not need to be inserted into the tumor after endoscopy (to be used to give him antibiotics and the desire continues within the next few days), while others are reported to place catheters in all cases but avoid drain implants in only one patient , and not a moment. surgery was needed because no tumors were left with the effect of replacement; on the contrary, they underwent subsequent surgery on their 4 patients. There are reports that no significant differences can be found in the length of hospital stay, the number of postoperative CT scans, and the duration of antibiotic treatment between traditional and endoscopic stereotactically guided aspiration. Internal sampling of abscess material operations and smear arrangements for microscopic analysis and biological detection of tumors is fraught with pitfalls.
First, tumor-related necrosis should be separated from tumor necrosis. Small or large areas of coagulation necrosis are often seen in glioblastomas. Sometimes the necrotic site of a tumor is replaced by a large infiltration of a polymorphonuclear leukocyte that transforms the necrotic area into a liquefactive, resulting in a misdiagnosis of brain tumor. On the other hand, perilesional gliosis of the abscess may be marked to mimic low-grade astrocytoma. Although in the non-neoplastic proliferation of active astrocytes cellular cells are usually low and individual cells are very common, they are rarely associated with cellular domains especially of the growing astrocytes with pleomorphic and hyper chromatic nuclei. Brain tumors were thus classified as cerebritis (StageI) when polymorphonuclear leukocyte deficiency and perivascular erythrocyte were recovered and encapsulation (StageII) in frank pus, a polymorphonuclear leukocyte.
constipation, necrosis, granulation tissue, and active active gliosis were detected; this provided a better understanding and simplification of pathological features as well as effective pathological-radiological communication.
On the other hand, advanced neuro radiological methods can be used in different diagnoses of these lesions. In deep, multi-site lesions, and near the ventricle wall, a reduction of 1 mm between the ventricle and brain tumors will increase the rate of fracture by 10%. Although a combination of intra-cal and intravenous antimicrobial therapy has been recommended for intraventricular rupture, treatment strategies in this specialized group of patients remain controversial. Alternative therapies have been recommended, including the following:
Outcome
The mortality rate ranged from 40 to 60% in the pre-CT period and decreased to 10% from the beginning of the CT period to 2000. After 2000, the mortality rate was reported to be between 17 and 32%. mainly due to the major changes in epidemiology occurring today.
Compared to previous reports, the incidence of brain tumors caused by sinus / otitis infection has decreased, while those associated with a deficiency of immune system have increased significantly. It is a challenge to treat patients who are receiving cancer chemotherapy or immuno-suppressive therapy for organ transplants, or who are infected with HIV. The author reported a 2.8-fold increased risk of adverse outcomes in patients with immuno-suppressive immunity. Other comorbidities such as diabetes mellitus or cirrhosis are also factors that have a negative effect on the outcome. Worst predictions were reported in patients presenting with low Glasgow Coma Scale scores. Than reported that (62%) of the 21 patients with the first figures for the Glasgow Coma Scale, 9 either died or fell into a vegetarian state. Intraventricular rupture is a traumatic and often fatal complication of a brain tumor and is associated with a high mortality rate.
Deaths were reported in (84.5%) of 129 patients in a literature review between 1950 and 1993. In another recent study from Japan, the overall mortality rate was 38.7% (12 of 31 patients). A series of 62 patients there (48%) had adverse effects (severe disability, vegetative status, and death) due to intraventricular rupture of brain abscess. The patient's neurological pre-treatment condition is an independent factor with a significant effect associated with the outcome. It is rare for survivors to experience neurological sequelae that include hemiparesis, epilepsy, and psychiatric disorders. Abduction is a long-term risk for 30-50% of patients suffering from brain tumors. The delay time can be up to 5 years, but it is short for older patients. started immediately and lasts for at least 1 year due to the high risk of subsequent fainting in patients with brain tumors.
Treatment may be discontinued if no significant epileptogenic activity can be demonstrated in electroencephalograms. Abscess management is one of the most important factors in both arousal and nerve effect. It was therefore reported that, after surgical removal of the abscesses, severe severe neurological deficits (5.2% compared to 0%) and fainting (47.7% compared to 31.2%) were observed compared with stereotactic cravings. The location of the abscess did not contribute to the formation of fainting. However, the hypodense areas around the tumor cavity were wider in patients treated with surgery. These areas were thought to be a damaged brain parenchyma that was causing neurological deficit and epilepsy. Recurrence rates are estimated at 10-50%. The observation period should be held for at least 1 year. The solution to the surrounding edema and the loss of the developing rim should be recorded at this time, which can take up to 6 months. in the first 6 weeks. Wounds that do not show a decrease should be sought as well. Surgical treatment may be recommended for patients with dementia and / or ulcers that cannot be resolved radiologically.