Journal of Urology and Nephrology Research
OPEN ACCESS | Volume 2 - Issue 1 - 2024
ISSN No: 3065-6699 | Journal DOI: 10.61148/3065-6699/JUNR
Colucci G*, Robusto F*, Colucci E*, Iacovazzo P*, Zamparella M*, Minardi M° Campana G*, Modoni A**, Mariano L°°, Colucci V^, Giliberti C.
*General Practitioner, °Chief Surgeon,** Head of Radiology, °°Head of Vascular Surgery, ^Biomedical Engineer, Italian Rice University, Houston, Texas, USA.
*Corresponding author: Colucci Giovanni, General Practitioner, °Chief Surgeon,** Head of Radiology, °°Head of Vascular Surgery, ^Biomedical Engineer, Italian Rice University, Houston, Texas, USA.
Received: April 15, 2025
Accepted: April 20, 2025
Published: April 26, 2025
Citation: Colucci G, Robusto F, Colucci E, Iacovazzo P, Zamparella M, Minardi M, Campana G, Modoni A, Mariano L, Colucci V, Giliberti C. (2025) “Benign Prostatic Hypertrophy (BPH): α-lytic, Testosterone-5-α-Reductase Inhibitors (5αRI) Alter the Glucose Profile in the Diabetic with Chronic Kidney Disease (CKD)”. Journal of Urology and Nephrology Research, 2(1); DOI: 10.61148/JUNR/050
Copyright: © 2024 Colucci Giovanni. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
5αRI and other drugs in the therapy of BPH may impair glucose metabolism (decompensated diabetes-DS),chronic kidney disease (CKD), androgens resulting in erectile dysfunction.Goal.To study the long-term adverse effects of therapy with G04CB02,G04CB01 in men with BPH on:a)glycated hemoglobin(EG); b)CKD; c)Arterial hypertension(AI); d)Obesity(OB);Materials and methods.Retrospective study.16 researchers(GPs) collaborated for a total of 24,000 patients.9502 men were identified during 2011 with a minimum follow-up period of 2 years.This cohort was followed for the following 7 years,verifying the possible appearance of CHD and DS in relation to exposure to 5αRI.Results.We divided the population into case (CS)[α-lytic-(GA04CA) -5αRI (GA04C)] 1356 and control(CT) 8146.Mean age ± SD:CS 68.0±14.5;CT: 47.7±16.2 (p<0.001);IA:CS 811(59.8%);CT:1816 (22.3%) (p<0.001);CKD:CS 64 (4.7%);CT 75 (0.9%) (p<0.001);OB:CS 296(21.8%);CT 726(8.9%) (p<0.001);DM:CS 308(22.7%);CT 496 (6.1%) (p<0.001);SD(EG>7):CS155(11.4%);CT 256(3.1%) (p<0.001).Cox adjusted for age,AI,OB,DM, EG on the possibility of developing CKD.On the contrary,no correlation was demonstrated. Creatinine(CR) measurement was performed at baseline and throughout the follow-up period in 4703 patients.Of these, 474 were on therapy with 5αRI. In these subjects there was an average decrease in the CR value of -0.11 mg/dL (95%CI -0.17 - - 0.05; p=0.0002) with an average percentage decrease of -14.87%(95%CI - 18.56% - -11.19%; p<0.0001).It can be roughly assumed that a 65-year-old patient with CR in the normal range has an eGFR of 75±15 ml/min and that this physiologically reduces by 0.8 ml/min for each subsequent year.Conclusions. 5αRI appear to worsen CKD.The General Practitioner must evaluate the GFR before administering the drugs and, based on the possible stage of the CKD, consider treatment with these drugs.
obesity; cardiovascular risk, chronic kidney disease
Introduction
Chronic renal failure (CKD) has acquired the characteristics of a global public health problem in recent decades (1). Based on the available registries, the prevalence of CKD in the general population varies between 7% and 13%, corresponding to a frequency that often exceeds that of type 2 diabetes (2). The almost pandemic spread of CKD is also in line with the increase in the global dimension of type 2 diabetes. In 2019, about 463 million people (9.3% of the global population) were affected by diabetes, of which 90% were type 2 diabetes.
It has been estimated that about 2 out of 5 patients with type 2 diabetes are affected by CKD at the same time (3). The incidence of diabetes-associated CKD (also known as 'diabetic kidney disease' DKD) has increased over the last 30 years in all age groups (4). It is known that CKD is a multifactorial pathological condition where multiple risk factors account for the patient's cardiorenal outcome. Among these, alongside eGFR and albuminuria, arterial hypertension, hyperkalemia, alterations in calcium-phosphorus metabolism, anemia, metabolic acidosis and dyslipidemia are the best known (5). At the same time, the aging of the male population, especially in the most developed countries, has led to a constant increase in the prevalence of subjects suffering from benign prostatic hypertrophy. The 5α-reductase (5α-RI) inhibitors, finasteride and dutasteride, are widely used in the treatment of lower urinary tract symptoms associated with benign prostatic hypertrophy (BPH) (6, 7). If, on the one hand, these drugs block the transformation of testosterone into dihydrotestosterone, lead to an improvement in the symptoms related to prostatic hypertrophy; on the other hand, they can slow down the metabolism and also lead to an increase in fat mass. Adipose tissue is now recognized to be a hormone-active tissue whose metabolic function is also carried out through the production of factors stimulating the production of glucocorticoids, primarily aldosterone. This mechanism could favor the establishment of iatrogenic hyperaldosteroinism secondary to the intake of 5α-RI. Aldosterone is a steroid hormone with mineralocorticoid activity, mainly produced in the glomerular area of the adrenal cortex. Its main physiological functions are to maintain sodium and potassium balance and control blood pressure, by binding to mineralocorticoid (MR) receptors in the distal tubule and collecting duct of the kidney, thereby increasing sodium reabsorption and potassium secretion (8). Aldosterone exerts multiple extrarenal effects including induction of inflammation, vascular rigidity, collagen formation and stimulation of fibrosis (9). There is now a strong body of evidence in favour of the role of aldosterone and MR receptor activation in the pathophysiology of cardiovascular and renal disease; clinical studies show the proven benefits of mineralocorticoid (MR) receptor antagonists on mortality and progression of cardiac and renal damage (10,11). The aim of the study is to evaluate at the population level the effect of chronic exposure to 5α-RI on renal function on a court of patients currently assisted by a cohort of General Practitioners (GPs).
Materials and Methods.
The clinical databases of 16 GPs operating in the provinces of Taranto and Foggia in Puglia were analyzed, selecting male patients over 40 years of age suffering from benign prostatic hypertrophy. This cohort was characterized at baseline in relation to clinical-demographic characteristics by specifically analyzing the presence/absence of arterial hypertension, obesity (BMI), diabetes mellitus, heart or renal failure. Blood chemistry tests were also recorded both at baseline and during follow-up such as glycosylated hemoglobin and serum creatinine. In addition, the cohort was stratified in relation to the use or not of 5α-RI. Subjects were followed for the next 7 years, excluding patients with less than 2 years of follow-up, evaluating whether there was a significantly greater worsening of glucose or renal metabolism in the population exposed to 5α-RI. For the study of CKD in the 5 KDOQI stages we applied the MDRD (Modification of Diet in Renal Disease) formula.
Statistical analysis
Data were presented as mean±SD and as percentage. Statistical analyses utilized T-test, Chi-square and Wilcoxon rank sum testing as appropriate. Incidence rates (IRs) with 95% confidence intervals of renal filtration rate were estimated during the follow-up period. Crude IR ratios (IRRs) were estimated using Poisson regression comparing patients exposed or not-exposed to 5α-RI. Risks were reported as IRRs along with their 95% confidence intervals and P values. Results were considered statistically significant when p<0.05. All statistical analyses were performed using SAS Software Release 9.4 (SAS Institute, Cary, NC).
Results.
From the population of 16 GPs in the province of Taranto, 20115 adult subjects were identified during the course of 2011 with at least 2 years of follow-up. The population affected by benign prostatic hypertrophy was 2518 men in charge of GPs in 2011 and with a follow-up period of at least 2 years and a regular creatinine measurement at least every 12 months. This cohort was followed for the next 7 years, verifying the possible occurrence of renal failure during the follow-up period in relation to chronic exposure to 5-alpha-reductase testosterone inhibitors. A total of 601 subjects with BPH who were chronically taking 5α- RI and 1917 subjects with BPH who were not taking this therapy were identified. The mean age of subjects exposed to 5α-RI was 72.8±11.2 years, while in controls 61.6±10.9 (p<0.001). As regards the baseline frequency of the main comorbidities, statistically significant differences (p<0.001) were recorded between the 2 groups with regard to arterial hypertension (55.6% vs 40.4%), diabetes mellitus (23.5% vs 14.2%); while the frequency of renal failure (1.5% vs 1.2%) and obesity (12.5% vs 11.8%) was not significantly different between the 2 groups. The baseline characteristics of the population are summarized in Table 1.
|
Features |
5-alpha-reductase inhibitors N° 601 |
Control N° 1917 |
p |
|
Age media±DS |
72.8±11.2 |
61.6±10.9 |
<0.001 |
|
High blood pressure No. (%) |
334 (55.6%) |
774 (40.4%) |
<0.001 |
|
IRC 4-5 england KDOQI No. (%) |
9 (1.5%) |
23 (1.2%) |
0.5697 |
|
Obesity N (%) |
75 (12.5%) |
227 (11.8%) |
0.6745 |
|
Diabetes mellitus No. (%) |
141 (23.5%) |
272 (14.2%) |
<0.001 |
|
compensated diabetes (HBG> N (%) |
42 (7.0%) |
109 (5.7%) |
0.2407 |
Table1: Characteristics of the population at baseline
The glomerular filtration value calculated at the baseline was 79.71±18.65 per person/year in subjects chronically exposed to 5- alpha-reductase inhibitors and 90.40±16.92 in the control group (p<0.0001). During follow-up, however, it dropped to 72.71±20.42 in subjects chronically exposed to 5-alpha-reductase inhibitors and 85.60±20.08 in the control group (p<0.001). There was therefore a mean percentage decrease of 8.8% (CI 6.5 – 10.2) in subjects chronically exposed to 5-alpha-reductase inhibitors and 5.3% (3.8 – 6.4) in the control (Figure 1). This decrease was also statistically significant when the results were corrected for the clinical- demographic factors analyzed at baseline (p<0.001).
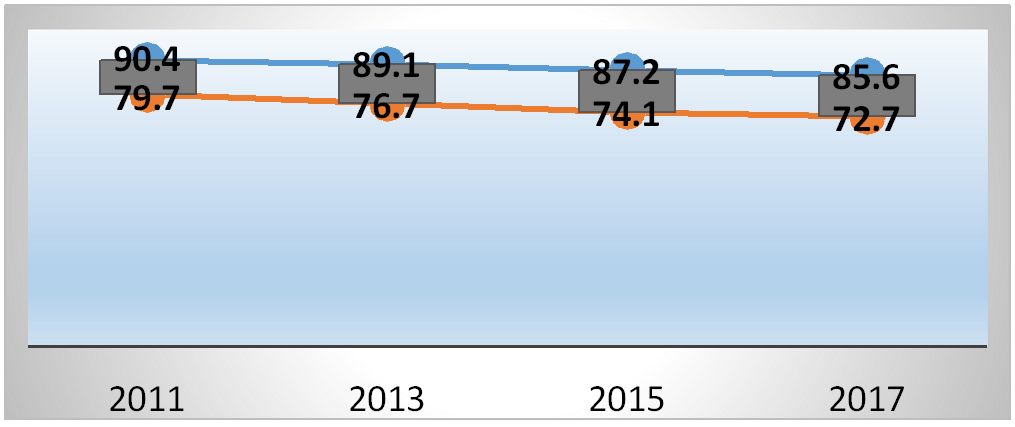
Figure 1: Trend of the glomerular filtrate value during the follow-up period
Discussion:
Dutasteride group vs. placebo group (0.7% [30 of 4105 men] vs. Recently, Upreti et al.[12] reported that inhibition of 5α-reductases (types 1 and 2) by dutasteride resulted in a reduction in glucose metabolism despite the infusion of high doses of insulin. The results of Joyce et al (13) confirmed previous data, among older men, where 5α-dihydrotestosterone (5α-DHT) levels were inversely associated with insulin resistance (IR) and diabetes risk. 5α-reductase receptors are highly expressed in the liver, but also in other tissues critical for insulin sensitivity, e.g. adipose tissue and skeletal muscle. Enzymes metabolize a number of steroids, including testosterone, cortisol, progesterone, and aldosterone. The increased susceptibility to type 2 diabetes could reflect changes in these hormones, most plausibly androgenic or glucocorticoids. 5α- dihydrotestosterone is a more active androgen than testosterone, so inhibition of its formation with dutasteride or finasteride (12) could induce androgen-deficiency characteristics, which include insulin resistance. Low circulating levels of testosterone are associated with an increased risk of type 2 diabetes (33) in men, a relationship that is also evident after androgen deprivation therapy in prostate cancer (14). The first studies of "heart failure" were by Andriole et al.(14) in their study of 6,729 men who underwent prostate biopsy or surgery, the dutasteride group had a higher composite relative incidence of heart failure than that observed in other studies. 0.4% [16 of 4126 men], p = 0.03; relative risk estimate, 1.91; 95% CI, 1.04-3.50)". Sean C Skeldon et al. studied 36,311 men exposed to dutasteride and 36,311 treated with finasteride on cardiovascular safety, conclusions Dutasteride was not associated with an increased risk of cardiovascular events compared to finasteride (15). In our court of about 20,000 patients suffering from arterial hypertension, type 2 diabetes mellitus, obesity and chronic kidney disease were included where glyco-metabolic imbalance was not demonstrated but a worsening of chronic kidney disease in the last stages, due to drugs. The province of Taranto is in first place, in Puglia, followed by Foggia with the highest incidence of Diabetes Mellitus and Obesity all related to air pollution and land pollution (the new land of Fires). Obesity can increase the risk of developing benign prostatic hypertrophy, a disease that affects more than 6 million Italians, by up to 40%. Estrogen has nowbeen recognized as one of the important regulators of prostate growth. The consumption of certain fatty acids, especially of animal origin, has been correlated with an increase in prostate problems. As adipose tissue is increasingly considered a hormone-active tissue, high body fat and obesity need in-depth exploration to understand the associated risk of prostate problems. It is now known that adipose tissue influences the circulating levels of several bioactive messengers and therefore could influence the risk of developing prostate problems in addition to many other well-known health problems. Fat cells in stored body fat secrete estrogen and a very high number (> 500) of biologically active substances called adipokines, as well as inducing, through other cell-driven effects, pathological changes in insulin pathways. Exposure to the hormonal and proinflammatory effects of excess adipose tissue are associated with an increased risk of 11 different cancers. 5α- reductases are highly expressed in the liver, but also in other tissues critical for insulin sensitivity, e.g. adipose tissue and skeletal muscle. Enzymes metabolize a number of steroids, including testosterone, cortisol, progesterone, and aldosterone. (Renal antifibrotic role of mineralocorticoid receptor antagonists). Activation of mineralocorticoid (MR) receptors is known to be a risk factor for heart disease (16); but aldosterone and MRs are also involved in various forms of kidney disease (diabetic and hypertensive nephropathy and various forms of glomerulopathies) (10). Aldosterone, through the activation of MRs, modulates the expression of proinflammatory cytokines and profibrotic factors, such as tumor necrosis factor-α, interleukin-1β, TGF-β (17, 18), osteopontin (19), fibrinogen and collagen type 1 (Figure 1) (4,21,22). With consequent damage to the heart-kidney, worsening cardiovascular risk. Aldosterone exerts multiple extrarenal effects including induction of inflammation, vascular rigidity, collagen formation and stimulation of fibrosis (22). Aldosterone plays a fundamental role in the pathophysiology of chronic heart failure and MRs are overexpressed in myocardiocytes of patients with congestive heart failure and in those with atrial fibrillation; in fact, overexpression of MRs induces ventricular remodeling and myocardial hypertrophy, development of cardiac damage due to reduced coronary flow and proarrhythmogenic effects (23,24). Activation of MRs also has negative effects on the kidney, such as glomerular hypertrophy, glomerulosclerosis, proteinuria, reduced renal plasma flow and glomerular filtrate, and thus chronic kidney injury (25,26).
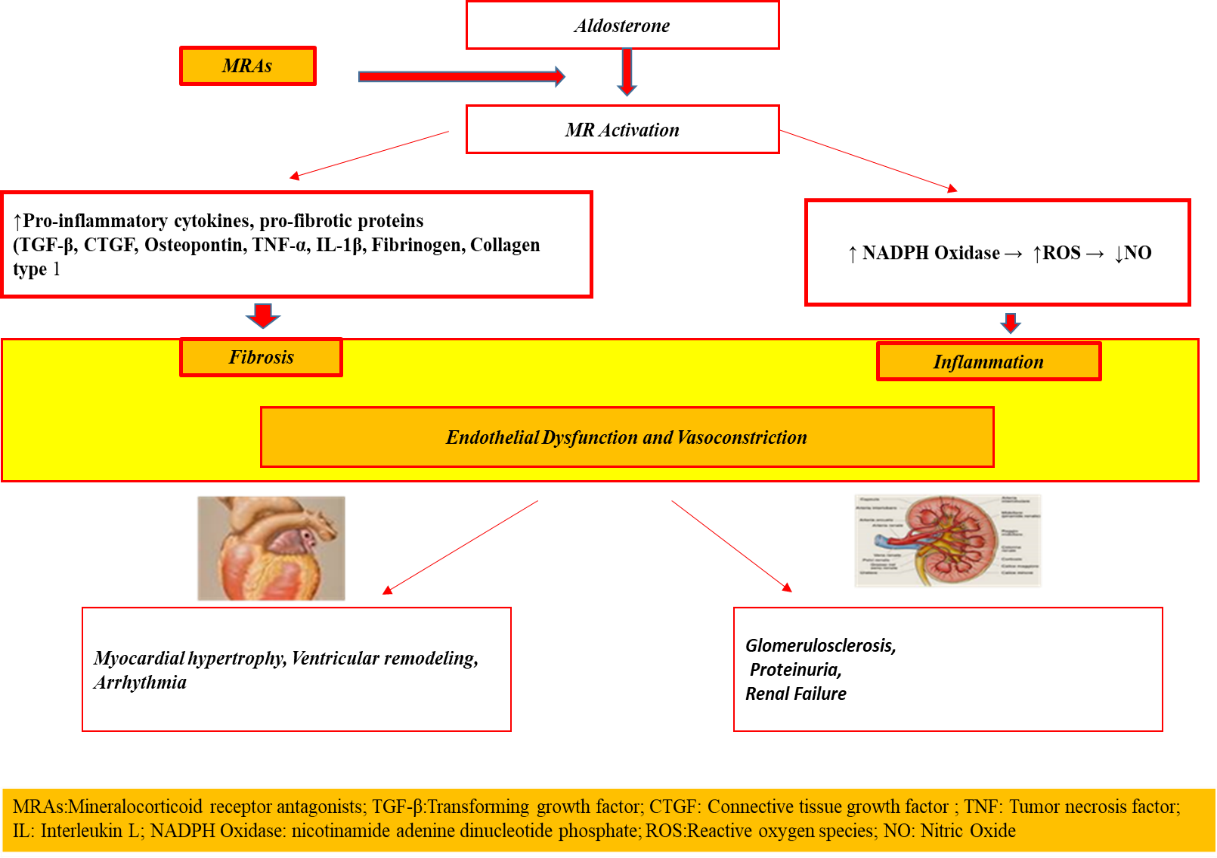
Figure 1: Cascade activation of cardiac and renal inflammatory processes.
In our study, with the administration of 5α-reductase (5α-RI) inhibitors, finasteride and dutasteride, for seven years we increased aldosterone with an annual reduction of minus 14% of glomerular filtrate, worsening the IV-V KDOQI (Kidney Disease Outcomes Quality Initiative) stages.
Conclusions.
In the prescription of these drugs, in the follow-up of the obese patient with diabetes, the cardio-renal risk must be controlled through laboratory tests for kidney and cardiac function. When comparing the various specialists, consider and/or suspend therapy, or administer aldosterone inhibitors.
Strengths and limitations of the study
The strengths of this study are the large number of patients, the long-term follow-up of 7 years, the inclusion of a comparison group, and the real-life setting (no inclusion or exclusion criteria) in which the effects of dutasteride/finasteride were clinically evaluated. Limits! encourage other working groups to confirm our data.