Journal of Urology and Nephrology Research
OPEN ACCESS | Volume 2 - Issue 1 - 2024
ISSN No: 3065-6699 | Journal DOI: 10.61148/3065-6699/JUNR
1Besong Elvis Obi, 2Salisu Usman, 3Shella Obi Besong, 4Ezeamii Victor Chiedozie, 5Oladosu Micheal Abimbola, *6Moses Adondua Abah, 1Akpotuzor David Uchendu, 7Possible Okikiola Popoola, 8Ali Sani Musa, 9Kelechi Wisdom Elechi, 10Tobi David Farinde, 11Olutayo Nathanael Farinde, 12Kelechi Asogwa, 13Taiwo Awojulu, 12Sunday Ameh, 6Ochuele Dominic Agida, 11Adedapo Olosunde
1Department of Haematology, Faculty of Medical Laboratory Science, University of Calabar, Nigeria.
2Department of Medical Laboratory Science, College of Medical Science, Faculty of Allied Health Science, Borno State University, Borno State, Nigeria.
3Department of Nursing Science, Faculty of Allied Health Sciences, University of Maiduguri, Borno State, Nigeria.
4Department of Biostatistics, Epidemiology and Environmental Health Sciences, Jiann-Ping Hsu College of Public Health, Georgia Southern University, USA.
5Department of Biochemistry, Faculty of Basics Medical Sciences, University of Lagos, Lagos State, Nigeria.
6Department of Biochemistry, Faculty of Biosciences, Federal University Wukari, Taraba State, Nigeria.
7Department of Physiology, Ladoke Akintola University of Technology, Oyo State, Nigeria.
8Department of Laboratory Services, Federal Neuropsychiatric Hospital Maiduguri, Borno State. Nigeria.
9Department of Integrated Biomedical Sciences, University of Texas Health Science Center, San Antonio, USA.
10Department of Human, Nutrition and Dietetics, Faculty of Public Health, University of Ibadan, Ibadan, Oyo state, Nigeria.
11Department of Chemistry & Biochemistry, University of Toledo, Toledo, OH 43606, USA.
12Department of Chemistry, Faculty of Physical Sciences, University of Benin, Edo State, Nigeria.
13Department of Chemical Engineering, Faculty of Engineering, University of Benin, Edo State, Nigeria.
*Corresponding author: Moses Abah Adondua, Department of Biochemistry, Faculty of Biosciences, Federal University Wukari, Taraba State, Nigeria.
Received: March 17, 2025
Accepted: April 10, 2025
Published: April 15, 2025
Citation: Besong Elvis Obi, Salisu Usman, Shella Obi Besong, Ezeamii Victor Chiedozie, Oladosu Micheal Abimbola. (2025) “Assessment of Copper and Nickel in Patients with Chronic Kidney Disease Attending University of Maiduguri Teaching Hospital (UMTH), Maiduguri, Borno State”. Journal of Urology and Nephrology Research, 2(1); DOI: 10.61148/JUNR/010
Copyright: © 2025 Moses Abah Adondua. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Chronic Kidney Disease (CKD) is a significant global health issue, affecting millions worldwide. The prevalence of impaired kidney function was estimated to range between 10% and 20% of the adult population in most countries. Chronic Kidney Disease (CKD) represents a progressing and life-threatening pathology which produces a series of changes in the bio-humoral parameters. Some of these changes that develop in CKD includes modify levels of minerals and trace elements such as copper and nickel. This study is aimed at estimating and evaluating the concentration of copper (Cu) and nickel (Ni) in patients with chronic kidney disease attending University of Maiduguri Teaching Hospital (UMTH), Maiduguri, Borno State. A total of 150 subjects were recruited for the study whereby 100 of the subjects were male and female patients attending University of Maiduguri teaching Hospital (UMTH) presenting with the signs and symptoms of kidney disease while the 50 were apparently healthy subjects used as control. At the kidney center and Neurology department, arrangement was made with the Physician whereby subjects who satisfy the study selection criteria were recruited. Informed consent for inclusion into the study was obtained from the subjects. The weight of the subjects were measured with a calibrated weighing balance with minimum clothing. The subjects were asked to stand upright on the scale facing forward. The weights were then determined by reading the scale and measured in kilogram (kg). The subjects were asked to stand up straight without any shoes or head covering beside the pole on horizontal surface. The heights were measured and recorded in meter (M). BMI was calculated from the formula; =weight in kg /height in m2. The systolic and diastolic blood pressures of the subjects were measured using a sphygmomanometer and stethoscope. Blood specimens (5ml each) were collected from ante-cubital vein using sterile disposable 5ml syringe and needle. The desired area for the sampling was selected and disinfected using methylated spirit and was allowed to dry. The blood was then collected by venipuncture and transferred to a plain container and allowed to clot at room temperature. It was centrifuged at 4,000 revolutions per minute (rpm) for 5 minutes and the sera were separated from the cells and transferred to a fresh sample container and stored frozen for batch analysis. The result revealed that there is significant difference (p< 0.05) in the means of serum copper (0.03 ± 0.02 mg/L vs. 0.05 ± 0.02 mg/L) and serum nickel (0.01 ± 0.01 mg/L vs. 0.04 ± 0.01 mg/L) of chronic kidney disease patients and controls. The result also shows that there is a partial positive and non-significant correlation (r = 0.141; p = 0.163) between Body Mass Index (BMI) and Serum Copper. Likewise, there is a partial positive and non-significant (r = 0.031; p = 0.756) correlation between Body Mass Index (BMI) and Serum Nickel. It also showed a partial negative significant correlation (r=-0.999; P=0.000,) between levels of copper and serum urea. There was also a weak negative significant correlation (r=-0.166; P=0.000) between levels of copper and serum creatinine. It also showed a weak positive significant correlation (r=0.086; P=0.000, r=0.089; P=0.000) between levels of nickel and serum urea and creatinine respectively. The study suggest that serum levels of copper and nickel were affected by the chronic kidney disease, serum levels of copper and nickel were low in patients with chronic kidney disease
chronic kidney disease (CKD); copper; nickel; kidney and patients
Introduction
Chronic kidney disease (CKD) is a global public health crisis that tends to take dimensions of epidemic and has severe impact on quality of patient’s life (Gerogianni and Babatsikou, 2014). It has a greater burden and prohibitive cost of care particularly in Maiduguri. It is a progressive, irreversible deterioration in renal function in which the body’s ability to sustain metabolic and fluid and electrolyte balance fails, resulting in uremia or azotemia (retention of urea and other nitrogenous wastes in the blood) (Leung, 2003; Ayo et al., 2023b). World Health Organization (WHO) statistics reveal that the death rate from intrinsic kidney and urinary tract disease was one million in the year 2002, ranking twelfth on the list of major causes of death (WHO, 2003). The prevalence of chronic kidney disease (CKD) in the community was grossly underestimated in the past. The prevalence of impaired kidney function was estimated to range between 10% and 20% of the adult population in most countries worldwide (WHO, 2003, Beaglehole and Yach, 2003). However, according to World Kidney Day, an estimated 10% of the population worldwide having CKD in 2015.
The kidney, a filtration apparatus for blood, is responsible for the removal of toxins, regulation of fluid, molecules and by-products of metabolic processes (Hall and Hall, 2011; Arowora et al., 2024; Asuelimen et al., 2024). Chronic Kidney Disease (CKD) is defined in (2012) by Kidney Disease Improving Global outcomes (KDIGO) as a gradual loss of kidney function or structural abnormality present for three months or longer, with an estimated glomerular rate (eGFR) <60 ml/min/1.73m2 and/or the presence of albuminuria (>30 mg), and that exerts influence or is influenced by trace elements circulating in the human body (Ojochenemi et al., 2019; Joseph and Abah, 2023).
Copper is an integral part of numerous enzymes systems; these copper-containing enzymes are fundamental to cellular respiration, free radical defense, neurotransmitter function, connective tissue synthesis, and iron metabolism (Fraga, 2005; Chellan and Sadler, 2015; Ayo et al., 2023); Oladosu et al., 2024). Changes in the serum concentration of copper in patients with renal insufficiency depend on many factors. However, the degree of renal insufficiency is an important determinant of its serum copper concentrations (Bogden and Klevay, 2000; Ife et al., 2024)).
Nickel is a nutritionally essential trace metal for several animal species, micro-organisms and plants and therefore either deficiency or toxicity symptoms can occur when too little or too much nickel is consumed (Cempel and Nikel, 2006). Nickel is important in the biological system, such as in enzyme activity and hormonal control, and also in RNA/DNA structure or function (Lu et al., 2005) Some studies have shown that the kidney serves as a major organ of nickel excretion and is a target organ for nickel toxicity due to nickel accumulation (Denkhausa, 2002 and Tyagi et al., 2013; Ayo et al., 2023a)).
This study is aimed at estimating and evaluating the concentration of copper (Cu) and nickel (Ni) in patients with chronic kidney disease attending University of Maiduguri Teaching Hospital (UMTH), Maiduguri, Borno State.
Materials and Method
Study Area
The study was conducted in the University of Maiduguri Teaching Hospital. University of Maiduguri was established in 1975 by the Federal Government of Nigeria as part of the Country’s third development plan (1975-1980). It is situated in the outskirt of Maiduguri along Bama Road (www.unimaid.org) while The University of Maiduguri Teaching Hospital (UMTH) is situated in Maiduguri town along Bama Road, Nigeria.
Study Subjects
A total of 150 subjects were recruited for the study whereby 100 of the subjects were male and female patients attending University of Maiduguri teaching Hospital (UMTH) presenting with the signs and symptoms of kidney disease while the 50 were apparently healthy subjects used as control.
Inclusion Criteria
The study only included both male and female patients who consented and attending UMTH with chronic kidney disease irrespective of age and also apparently healthy individual who have not been diagnosed with kidney disease was included as control. Informed consent for inclusion into the study was sought from the selected students using a standard protocol (Appendices I and II).
Exclusion Criteria
Patients without kidney disease signs and symptoms irrespective of age and patients with known history of kidney disease were excluded from the study. Subject who declined consent on medication and other illness were excluded from the study.
Ethical Consideration
Ethical approval was obtained from Ethical Committee of UMTH, Maiduguri in accordance with Helsinki Declaration. This is a code of ethics on human experimentation drafted by the World Medical Association in 1964.
Sample Size Determination
The desired sample size was determined using Fisher, 1998 formula (Fisher, 1998).
Sample size (n) is given by the formula;
n = (z1 – a)2 (µ) (1 - p)
d2
Where n = minimum sample size, (z1 – a)2 = value of standard normal deviation which at 95% confidence level has been found to be 1.96,p = the best estimate of the population prevalence obtained from the literature review, d = difference between the true population rate and sample that can be tolerated, that is absolute precision required (in percentage point) on either site of the population.
Data: Z1=1.96
P=7.0%
d=5.0%
The current prevalence of the Kidney disease in Maiduguri is 7% (Odubanjo et al., 2011).
Therefore,
n = (1.96)2 (0.05) (1 – 0.05)
(0.05)2
n =3.8416x0.07x0.930.0025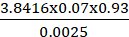
n = 0.250088160.0025
n = 100
The calculated sample size is 100.
Sampling Techniques
At the kidney center and Neurology department, arrangement was made with the Physician whereby subjects who satisfy the study selection criteria were recruited. Informed consent for inclusion into the study was obtained from the subjects. The nature of the study was explained to the subject using an appropriate language. A full medical history was obtained from the subjects followed by anthropometric and blood pressure measurements and collection of blood specimen. The findings were documented.
Anthropometric Measurements
The parameters measured include Height, Weight and Body Mass Index (MBI)
Weight
The weight of the subjects was measured with a calibrated weighing balance with minimum clothing. The subjects were asked to stand upright on the scale facing forward. The weights were then determined by reading the scale and measured in kilogram (kg).
Height
The subjects were asked to stand up straight without any shoes or head covering beside the pole on horizontal surface. The heights were measured and recorded in meter (M).
Body Mass Index (BMI)
BMI was calculated from the formula; =weight in kg /height in m2.
Measurements of Blood Pressure
The systolic and diastolic blood pressures of the subjects were measured using a sphygmomanometer and stethoscope.
Blood Specimen Collection and Processing
Blood specimens (5ml each) were collected from ante-cubital vein using sterile disposable 5ml syringe and needle. A tourniquet was applied to the upper arm (about 2-3cm above the ante-cubital region). The desired area for the sampling was selected and disinfected using methylated spirit and was allowed to dry. The blood was then collected by venipuncture and transferred to a plain container and allowed to clot at room temperature. It was centrifuged at 4,000 revolutions per minute (rpm) for 5 minutes and the sera were separated from the cells and transferred to a fresh sample container and stored frozen for batch analysis.
Estimation of Serum Urea
Serum urea was estimated using diacetylmonoxime (DAM) chemical method (Ormsby, 1942).
Estimation of Serum Creatinine
Serum creatinine concentrations were estimated using Jaffe’s method, (1886)
Estimation of Serum Bicarbonate
Serum bicarbonate was measured using acid-based titration method (Van Slyke et al., 1919)
Estimation of Serum Chloride
Serum chloride was measured using Schales and Schalles method (Schales and Schalles, 1941)
Estimation Of Serum Sodium and Potassium
Serum sodium and potassium was measured using flame emission photometric method (Hald, 1947)
Estimation of Serum Copper and Nickel
Serum copper and nickel were measured using atomic absorption spectrophotometric method (Walsh, 1955)
Statical Analysis
The data obtained were analysed using statistical package of social science (SPSS) version 16. The results of serum urea, creatinine, sodium, potassium, bicarbonate, chloride, copper and nickel obtained from kidney disease patients and controls were compared using one way analysis of variance (ANOVA). Where there was significant difference, a post-hoc analysis was carried out using student t-test statistical method. Correlations of BMI with mentioned biochemical parameter were carried out using Pearson linear correlation analysis. A p-value of less than 0.05 (p ˂ 0.05) was considered as significant
Results
Demographic Data of CKD Patients and Control Subjects
Table 1 shows the demographic distribution of both chronic kidney disease patients and controls. The result revealed that there is a significant difference (p< 0.05) in the means of ages of chronic kidney disease patients and control subjects. The result shows a significant difference in the BMI of the Chronic Kidney Disease (CKD) patients and the controls.
Table 1. Demographic data of CKD patients and control subjects
|
Variables |
CKD Patients |
Controls |
p-value |
Remarks |
|
|
Age (years) |
48.78 ± 13.320 |
41.66 ± 5.22 |
0.000 |
S |
|
|
BMI (kg/m2) |
26.70 ± 4.41 |
21.83 ± 1.92 |
0.000 |
S |
|
|
Gender |
Males |
54 |
41 |
- |
- |
|
Females |
46 |
9 |
- |
- |
|
Values are presented as mean ± standard deviation
Key:
S= there is a statistically significant difference between the means
NS= No statistically significant difference between the means
Comparison of Mean of Cu and Ni levels of CKD Patients and Controls
Table 2 shows the comparison of mean of copper and nickel levels of chronic kidney disease patients and controls. The table revealed that there is significant difference (p< 0.05) in the means of serum copper (0.03 ± 0.02 mg/L vs. 0.05 ± 0.02 mg/L) and serum nickel (0.01 ± 0.01 mg/L vs. 0.04 ± 0.01 mg/L) of chronic kidney disease patients and controls.
Table 2. Comparison of Mean of Cu and Ni levels of CKD Patients and Controls
|
Variables |
CKD patients (n=100) |
Controls (n=50) |
t-value |
p-value |
Remarks |
|
Serum Copper (mg/L) |
0.03 ± 0.02 |
0.05 ± 0.02 |
-13.5 |
0.000 |
S |
|
Serum Nickel (mg/L) |
0.01 ± 0.01 |
0.04 ± 0.01 |
-43.5 |
0.000 |
S |
Values are expressed as mean ± standard deviation
Key:
S= there is a statistically significant difference between the means
NS= No statistically significant difference between the means
Correlation Between the BMI with Cu and Ni levels of CKD Patients and Controls
Table 3 shows Pearson’s correlation of Body Mass Index (BMI) with Serum Copper and Serum Nickel. There is a partial positive and non-significant correlation (r = 0.141; p = 0.163) between Body Mass Index (BMI) and Serum Copper. Likewise, there is a partial positive and non-significant (r = 0.031; p = 0.756) correlation between Body Mass Index (BMI) and Serum Nickel.
Table 3. Correlation between the BMI with Cu and Ni levels of CKD Patients and controls
|
Variable |
Pearson’s Correlation Coefficients (r) |
p-value |
Remarks |
|||
|
|
CKD patients |
control |
CKD patients |
Control |
CKD patients |
control |
|
BMI and Cu (mg/L) |
0.141 |
0.105 |
0.163 |
0.468 |
A partial positive correlation but NS |
A partial positive correlation but NS |
|
BMI and Ni (mg/L) |
0.031 |
0.171 |
0.756 |
0.236 |
A partial positive correlation but NS |
A partial positive correlation but NS |
Key:
S= there is a statistically significant correlation between the two variables
NS= No statistically significant correlation between the two variables
Correlation Between Copper and Biochemical Parameters in Chronic Kidney Disease Patients
Table 4 shows the correlation between the levels of copper with the biochemical parameters of CKD patients. It showed a partial negative significant correlation (r=-0.999; P=0.000,) between levels of copper and serum urea. There was also a weak negative significant correlation (r=-0.166; P=0.000) between levels of copper and serum creatinine.
Table 4. Correlation between Copper and biochemical parameters in Chronic Kidney Disease Patients
|
Parameters |
Pearson’s Correlation Coefficients (r) |
P-value |
Remarks |
|
Urea(mmol/L) |
-0.999 |
0.000 |
S |
|
Creatinine(µmol/L) |
-0.166 |
0.000 |
S |
Key:
S= there is a statistically significant correlation between the two parameters
NS= No statistically significant correlation between the two parameters
Correlation between Nickel and Biochemical Parameters in Chronic Kidney Disease Patients
Table 5 shows the correlation between the levels of Nickel with the biochemical parameters of CKD patients. It showed a weak positive significant correlation (r=0.086; P=0.000, r=0.089; P=0.000) between levels of nickel and serum urea and creatinine respectively.
Table 5. Correlation between Nickel and Biochemical Parameters in Chronic Kidney Disease Patients
|
Parameters |
Pearson’s Correlation Coefficients (r) |
P-value |
Remarks |
|
Urea(mmol/l) |
0.086 |
0.000 |
S |
|
Creatinine(µmol/l) |
0.089 |
0.000 |
S |
Key:
S= there is a statistically significant correlation between the two parameters
NS= No statistically significant correlation between the two parameters
Correlation Scattered Plot Between Serum Urea and Copper in CKD patients
Figure 1 shows the correlation between serum urea and Copper in CKD patients. The result obtain showed a partial negative significant correlation between levels of copper and serum urea .

Figure 1: Correlation scattered plot between serum urea and Copper in CKD patients
Correlation Scattered Plot Between Serum Creatinine and Copper in CKD Patients
Figure 2 shows the correlations between Serum Creatinine and Copper in CKD patients. The plot shows a weak negative significant correlation between levels of copper (Cu) and serum creatinine

Figure 2: Correlation scattered plot between serum creatinine and copper in CKD patients
Correlation Scattered Plot Between Serum Urea and Nickel in CKD Patients
Figure 3 shows the correlation between Serum Urea and Nickel in CKD patients. It showed a weak positive significant correlation between levels of nickel and serum urea respectively.

Figure 3: Correlation scattered plot between serum urea and Nickel in CKD patients
Correlation Scattered Plot Between Serum Creatinine and Nickel in CKD patients
Figure 4 shows the correlation between Serum Creatinine and Nickel in CKD patients. It showed a weak positive significant correlation between levels of nickel and serum Creatinine respectively.

Figure 4: Correlation scattered plot between serum Creatinine and Nickel in CKD patients
Discussion
Chronic Kidney Disease (CKD) represents a progressing and life-threatening pathology which produces a series of changes in the bio-humoral parameters. Some of these changes that develop in CKD includes modify levels of minerals and trace elements such as copper and nickel. In a normal levels, these trace elements play an essential role in healthy organisms, however, in CKD because of reduced glomerular filtration rate, structural abnormalities, disturbance in acid-base status, modified levels of these trace elements might produce alteration in metabolism, enzymes activity and also membrane potential imbalance, which may induce cardiac arrhythmia and sudden death (Faur et al., 2020).
In this study, the mean of the serum levels of copper (0.03 ± 0.02 mg/L vs. 0.05 ± 0.02 mg/L) were low in CKD patients than in control subjects (table 2) and it is statistically significant (p-value < 0.05) which agree with the findings of Kaminska-Galwa et al., (1993) and Anees et al., (2011) who reported that serum copper levels were significantly deficient in CKD patients however it is inconsistent with the study of Shih et al., (2013) who reported significantly high levels of serum copper, however, Ongajooth et al., (1996) reported no significant effect on serum copper levels in their studies. This result showed inconsistency with the findings of Atlihan et al., (1991) and Lin et al., (1996) who reported in their findings higher levels of serum copper in hemodialysis patients in comparison with control subjects. In another study by Hsieh et al., (2006), they reported that there were no difference in copper levels between CKD patients undergoing dialysis and control subjects.
The present study (table 2) showed low mean serum levels of nickel (0.01 ± 0.01 vs. 0.04 ± 0.01) in CKD patients in comparison with control subjects and it is statistically significant (p-value < 0.05) which disagrees with findings of Hsieh et al., (2006) who reported that CKD patients undergoing hemodialysis had significantly higher levels of nickel in comparison with control subjects. According to Klatka et al., (2015) high level of nickel is found in patients with end stage renal disease (ESRD) leaving in region with high nickel exposure such as welding areas.
Chronic kidney disease patients are at theoretically risk for both accumulation and deficiency of copper and nickel depending on dietary intake, removal by dialysis, the composition of some water used for hemodialysis and residual kidney function (D’haese and De Broe, 1996). According to Prasad, (2013) serum copper level reduced in end stage renal disease (ESRD) due to protein and albumin excretion in urine.
However, table 3 of this study showed a partial positive correlation between BMI and copper levels of CKD patients and control subjects but is statistically insignificant. This showed that there is a partial positive relationship between BMI and levels of serum copper, as BMI increases serum copper level also increases in same direction with BMI. According to World Health Organization (WHO) a BMI between 18.5 and 25 kg/m2 is considered to be normal weight, a BMI between 25 and 30 kg/m2 as overweight, and a BMI of >30 kg/m2 as obese. Kramer et al., (2005) reported that overweight (BMI 25–29.9 kg/m2) and obesity (BMI ≥30 kg/m2) were associated with CKD, defined as the presence of +1 or greater proteinuria and/or eGFR <60 mL/min/1.73 m2. Numerous large population-based studies have shown that, higher level of BMI is associated with the presence and development of low estimated GFR with more rapid loss of estimated GFR over time, and with the incidence of end stage renal disease (Gelber et al., 2005, Ejerblad et al., 2006 and sVivante et al., 2012). In addition, there are various studies that linked elevation of serum copper and obese (Kahn and Flier, 2000, Omar et al., 2001, Lima et al., 2006 and Fan et al., 2017).
The present study (table 3) showed a partial positive correlation between BMI and levels of serum nickel of CKD patients and control subjects. The relationship between serum nickel and BMI is statistically insignificant.
This study found a significant partial negative correlation between levels of copper and serum urea in CKD patients with a weak negative significant correlation between copper levels and serum creatinine (table 4). This is in disagreement with the findings of Balla and Ismail, (2016) who reported that no correlation between serum copper levels and serum urea and creatinine in hemodialysis patients.
The present study found a significant weak positive correlation between levels of nickel and serum urea and creatinine respectively (table 5). Although this study of correlation between nickel levels and serum urea and creatinine is probably the first study, therefore could not relate with any study, more research is needed to ascertain this claim in this study.
Conclusion
In conclusion, serum levels of copper and nickel were affected by the chronic kidney disease, serum levels of copper and nickel are low in patients with chronic kidney disease. The decreased in both levels of serum copper and nickel in patients with CKD are statistically significant. There is partial positive correlation between the BMI with serum levels of copper and nickel of CKD patients and controls, therefore, modifying lifestyle and diet is paramount in monitoring CKD. Also, the meals for CKD patients should be fortified with basic trace elements to avoid deficiency or insufficiency.
Acknowledgments
We want to thank all the researchers who contributed to the success of this research work.
Conflict of Interest
The authors declared that there are no conflicts of interest.
Funding
No funding was received for this research work.