Journal of Dermatology and Venereology
OPEN ACCESS | Volume 4 - Issue 1 - 2026
ISSN No: 3065-677X | Journal DOI: 10.61148/3065-677X/JDV
Wenhui Huang1*, Lifang Gao2, Huanling Yu2, Peiyuan Fan1, Le Chen1, Zupeng Zou1, Rui Liu3, Zhengnan Liu4
1Department of Dermatology, Fuxing Hospital Affiliated to Capital Medical University, Beijing 100045.
2School of Public Health, Capital Medical University, Beijing 100069.
3Xuanwu Hospital, Capital Medical University, Beijing 100053.
4Yanjing Medical College, Capital Medical University, Beijing 101300.
*Corresponding author: Wenhui Huang, Department of Dermatology, Fuxing Hospital Affiliated to Capital Medical University, Beijing 100045.
Received: June 15, 2025 |Accepted: July 23, 2025 |Published: August 04, 2025
Citation: Huang W, Gao L, Yu H, Fan P, Chen L, Zou Z, Liu R, Liu Z., (2025) “Clinical Study on the Improvement of Facial Skin Condition by Oral Solution of Collagen Peptide.” Journal of Dermatology and Venereology, 3(1); DOI: 10.61148/3065- 677X/JDV/034.
Copyright: © 2025 Wenhui Huang. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Purpose: To investigate the effects of two collagen peptide oral liquids on improving the facial skin condition of subjects.
Methods: A total of 142 eligible subjects aged 35-45 years were recruited and randomly assigned into two groups: the collagen peptide EGCG group and the collagen peptide vitamin C group. Subjects consumed 50 ml of collagen oral solution daily for 90 days. Physiological and imaging data of the subjects' facial skin were collected and measured before the intervention and after the intervention period.
Results: After consumption of the two products, the moisture content of the facial skin stratum corneum significantly increased compared to pre- intervention parameters, with a significant improvement in skin moisture (p<0.001). The transepidermal water loss (TEWL) at both cheek test points was significantly reduced (p<0.01). The rebound time of the skin on the left and right cheeks decreased, although there was no statistical difference (p > 0.05). Imaging results showed that superficial and deep pigmentation, the number of pores, the number of skin wrinkles, and the index values of red area, brown area, and porphyrin all decreased, with a significant improvement in skin smoothness (p<0.001).
Conclusion: The oral solution of collagen peptide can enhance skin moisture, improve skin barrier function, inhibit endogenous pigment production in the skin, and promote anti-inflammatory, anti-wrinkle, and skin metabolism effects.
Collagen is the most widely distributed protein in the human body, accounting for approximately 70% of the skin's dry weight. After the age of 25, the collagen content in the dermis decreases annually, leading to gradual water loss and skin aging. Currently, dietary and oral supplements are important methods for improving skin appearance and structure. Skin is influenced by dietary substances, including vitamins, antioxidants, fatty acids, and hydrolyzed proteins, and healthy skin largely reflects overall health [3-5].
Collagen peptide is produced through the enzymatic hydrolysis of collagen. With its low molecular weight and high digestibility, collagen peptide has long been used as a supplement for improving skin and cartilage tissue. After being absorbed by the digestive tract, collagen peptide partially exists in the blood as small peptides and accumulates in the skin for up to 96 hours. Collagen peptides have beneficial functions in the skin, exhibiting antioxidant, anti- inflammatory, and promoting fibroblast chemotaxis, migration, and proliferation effects. Recent studies have reported that collagen peptide supplementation can effectively increase the moisture of the skin stratum corneum, improve the living environment of skin cells, promote skin metabolism, improve circulation, delay aging, and moisturize the skin.
The purpose of this study was to evaluate the improvement of facial skin physiological and aging-related skin conditions in women aged 35 to 45 years. Wrinkles, pores, pigmentation, red inflamed areas, and other skin conditions were assessed for the effects of collagen peptide oral solution on facial skin.
Materials and Methods Research
objects
This study was approved by the Ethics Committee of the Affiliated Fuxing Hospital of Capital Medical University (Batch No.: 2022FXHEC-KY059; clinical trial registration number: CHiCTR2300069080). All subjects provided informed consent. From November 2022 to February 2023, 142 volunteers were recruited and assigned to the collagen EGCG oral liquid group (referred to as the collagen peptide EGCG group), comprising 72 cases, or the collagen peptide vitamin C oral liquid group (referred to as the collagen peptide vitamin C group), comprising 70 cases.
Inclusion and Exclusion Criteria Inclusion Criteria are shown as follows:
Exclusion Criteria:
Research Method
Test Samples
Collagen peptide EGCG oral liquid, containing 6,000 mg of collagen peptide, 100 mg of EGCG, 100 mg of hyaluronic acid, and 500 mg of chitooligosaccharide; collagen peptide vitamin C oral liquid, containing 5,000 mg of collagen peptide and 250 mg of vitamin C. Both oral liquids have a specification of 50 ml per bottle and were provided by Beijing Qingyan Boshi Health Management Co., Ltd. The appearance, shape, color, and smell of the two products are not significantly different. The dosage is once daily, 50 ml each time, for 90 days.
Skin Physiological Index Detections
The skin physiological indicators of the subjects' face were collected using the CORTEX Combo (Cortex Technology, Denmark) skin tester at 0 days and 90 days after the intervention, including the moisture content of the skin stratum corneum (hydration/moisture), transepidermal water loss (TEWL), and skin elasticity [12].
Detection of Moisture Content in the Skin's Stratum Corneum Eight points on the subject's face were collected, located at the forehead (SHUI1), the left (SHUI2) and right (SHUI3) corners of the eyes, the left (SHUI4) and right (SHUI5) cheeks, and the left (SHUI6) and right (SHUI7) mouth corners, as well as the chin (SHUI8). SHUI9 represents the average moisture content among each point.
Testing of TEWL
Four points on the face of each subject were collected at the left (T1) and right (T2) corners of the eye and left (T3) and right (T4) cheeks.
Testing of Skin Elasticity
In this study, the skin elastic modulus (Young's elasticity modulus, E/MPa), skin retraction time (R/ms), and skin viscoelasticity (ViscoElasticity, VE/MPa) were used as skin elasticity detection parameters [13]. Two points on the left and right cheeks of each subject were collected.
Skin Imaging Testing
A multispectral skin mirror image processing station (Cloud mirror, CBS-2021, Wuhan Boshi Electronics Co., Ltd.) was used for skin image collection and testing, utilizing 8 spectral imaging to obtain detection indicators such as skin pigmentation, wrinkles, and pores. Data were collected for each subject at 0 days of intervention and 90 days after intervention, and facial photography and quantitative analyses were performed to comprehensively analyze the total area, density, and intensity of skin lesions. The detection items included pigment, including surface pigment RGB (mm2), dark pigment Deep (mm2), number of skin pores, superficial wrinkles, fine lines, deep wrinkles, red areas (representing skin inflammation), red area concentration (%) (representing inflammation severity), and porphyrin [14-16].
Safety Evaluation
We collected and recorded the incidence (%) and frequency (number of events) of adverse events (AEs) and serious adverse events (SAEs) occurring during the test. We analyzed the correlation with the trial products and recorded the treatment methods and results.
Statistical Analysis
The SPSS 20.0 statistical analysis software was used to analyze the data. Paired samples were statistically analyzed before and after the test for the two products. The results before and after treatment of the same subject were compared, i.e., the subject was paired before and after the interventions. All outcome variables were continuous, and the differences were in accordance with a normal distribution or approximately normal distribution. For differences that did not match the measurement data of normal distribution, the results were described by the median and interquartile range (P75).
Significance differences are represented by *(P<0.05), **(P< 0.01),***(P< 0.001) and ****(P<0.0001).
Results
Basic information of the subjects A total of 142 female subjects were enrolled in this study, aged 35 to 45 years (39.61±3.29). In the collagen peptide EGCG group, 12 cases were lost to follow-up, and 60 cases were included in the statistics. In the collagen peptide vitamin C group, 10 cases were lost to follow-up, and 60 cases were included in the statistics.
Skin Physiological Index Results
The moisture content of the skin stratum corneum increased in the collagen peptide EGCG group after 90 days, with a significant difference (p<0.001). The change rate of the 8 test sites ranged from 23.66% to 51.76%, with an average change rate of 33.23%. The change rates of the forehead and eye corner were much larger than the average change rate of the cheeks. The mean water content of the 8 test sites and the whole face stratum corneum (SHUI 9) in the collagen group increased significantly compared with before intervention. In the collagen peptide vitamin C group, the change rate of the 8 sites ranged from 14.91% to 48.83%, with an average change rate of 30.10%. The change rates of water content in the corneum of the forehead and right corner of the eye reached 48.83% and 46.33%, respectively, which were significantly higher than the average change rate of water content in the whole area (SHUI9). These results indicate that both oral liquids have the effect of increasing the moisture content of facial skin stratum corneum.
TEWL Results
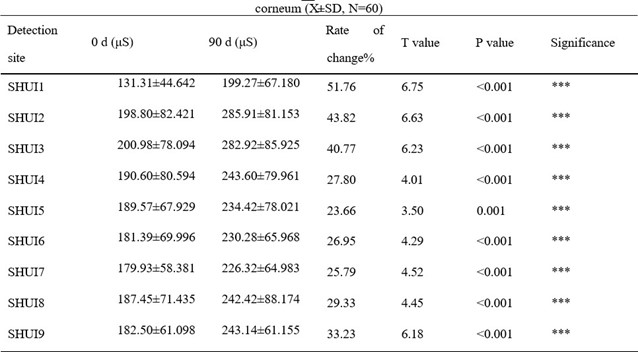
Table 1: The effect of collagen peptide EGCG oral solution on the moisture content of skin stratum
*** indicates comparison with 0 d, p < 0.001
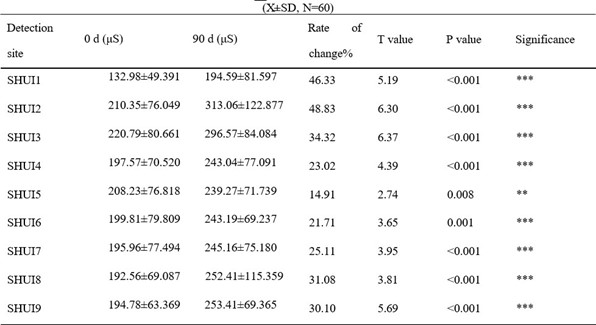
Table 2: The effect of collagen Vitamin C oral solution on the moisture content of skin stratum corneum
* indicates comparison with 0 d, p < 0.05 ;** indicates comparison with 0 d,p < 0.01 ;*** indicates comparison with 0 d, p < 0.001
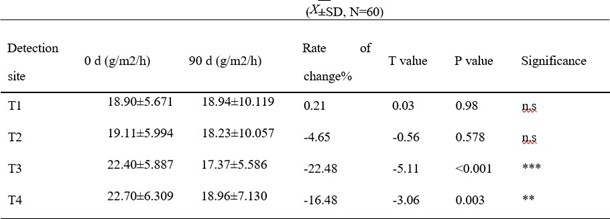
Table 3: Effects of collagen peptide EGCG oral solution on percutaneous water loss
* means comparing with 0 d p < 0.01; * * *, p < 0.001; ns means comparing with 0 d,p > 0.05, no significant difference
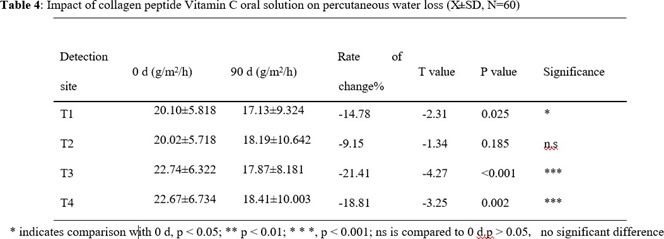
TEWL results are shown in Tables 3 and 4. In the collagen peptide EGCG group, TEWL on both cheeks (T3, T4) decreased compared to that before intervention. The left cheek (T3) change rate was - 22.48%, and the right cheek (T4) change rate was -16.48%. There was no significant difference in the changes of skin TEWL in the bilateral T1, T2 (p>0.05). In the collagen peptide vitamin C group, the TEWL of both cheeks (T3, T4) decreased significantly compared with that before intervention. The left cheek (T3) change rate was -21.41%, and the right cheek (T4) change rate was -18.81%. TEWL of both T1 and T2 decreased after intervention, with the left corner (T1) showing a significant difference from that before intervention (p<0.05). There was no significant difference in TEWL in the right corner of the eye (T2) compared with that before intervention (p > 0.05). The above results suggest that the two oral liquids have improved the barrier function of the stratum corneum in different parts of the facial skin.
Skin Elasticity Index Results
The test results of skin elasticity index are shown in Table 5 and 6. In the collagen peptide EGCG group, the skin elasticity of the subjects increased at the two test points of the cheeks, with a change rate of 5.03% on the left cheek and 8.52% on the right cheek. However, there was no statistical significance (p>0.05). After the intervention, the value of skin elastic modulus increased by 1.50% and 2.78%, respectively, with the right cheek showing a significant difference from that before the intervention (p<0.05). The time of skin retraction on the left and right cheeks decreased after intervention, with change rates of -3.45% and -8.78%, respectively, but there was no statistical significance (p > 0.05). In the collagen peptide vitamin C group, the viscoelasticity value of both cheeks increased compared to before intervention, with a change rate of 4.90% on the left cheek and 6.94% on the right cheek, but there was no statistical significance (p > 0.05). After intervention, the value of skin elastic modulus increased by 1.54% and 0.94%, respectively, with no significant difference compared to before intervention (p > 0.05). The time of skin retraction on the left and right cheeks decreased after intervention, with change rates of -7.95% and -5.18%, respectively, but there was no statistical significance (p > 0.05). The above results suggest that the collagen peptide oral liquids tend to improve skin elasticity.
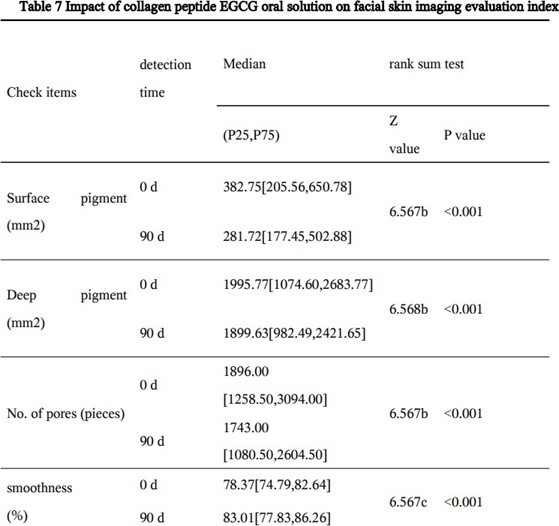
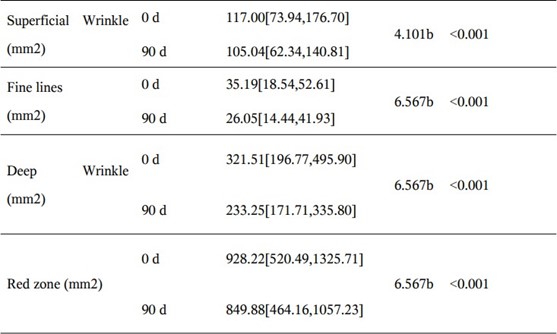
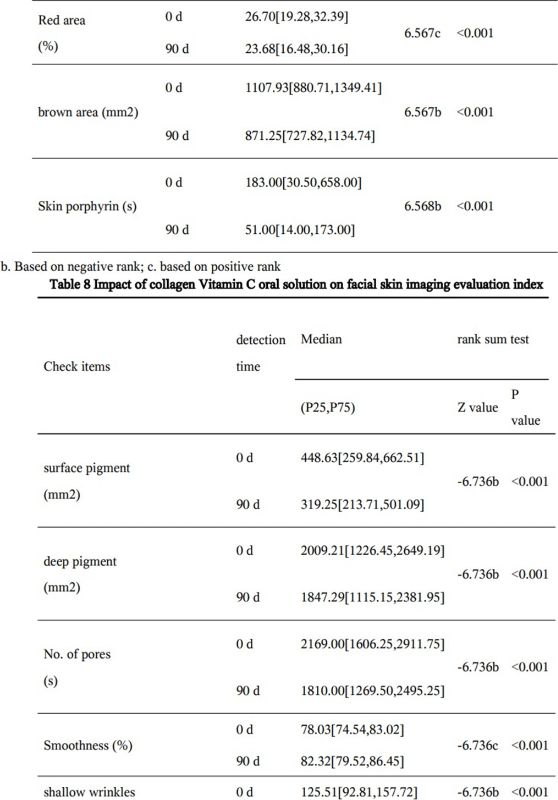
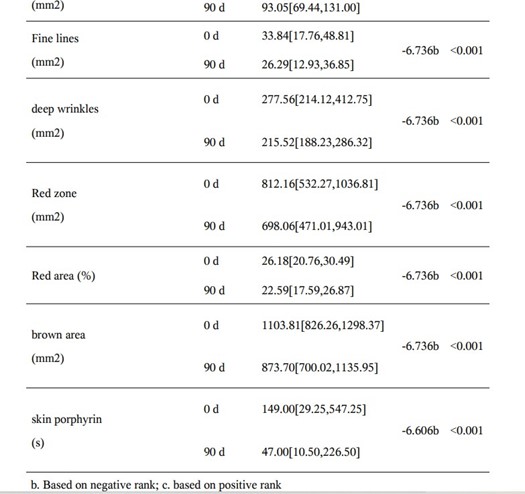
Skin Imaging Results
The results of skin imaging are shown in Table 7 and 8. After intervention, the surface pigment and deep pigment of the subjects were significantly reduced with significant differences (p<0.001), hinting at a whitening effect. The number of skin pores, superficial wrinkles, fine lines, and deep wrinkles, red areas, brown areas, and porphyrins were significantly reduced, and the smoothness of the skin was significantly improved, with significant differences (p<0.001). The above results show that the two collagen oral liquids have anti-wrinkle, whitening, improving skin metabolism, and anti-inflammatory effects. Typical pictures are shown in Figure 1 and Figure 2, illustrating the beneficial effects of collagen peptide on skin inflammation and redness
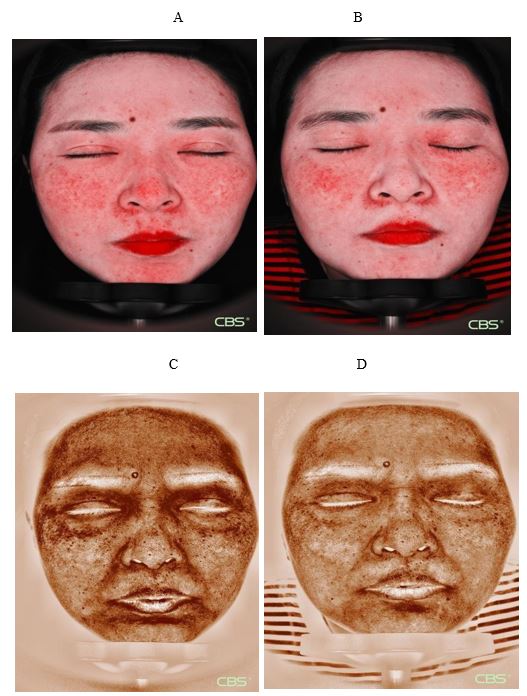
Figure 1: Cloud mirror picture of typical subjects in the collagen peptide EGCG group Inflammatory characteristic pattern imaging: Inflammatory reduction. A: 0 day
B: 90 day
Brown mode imaging: The pigment is light. C: 0 day D: 90 day
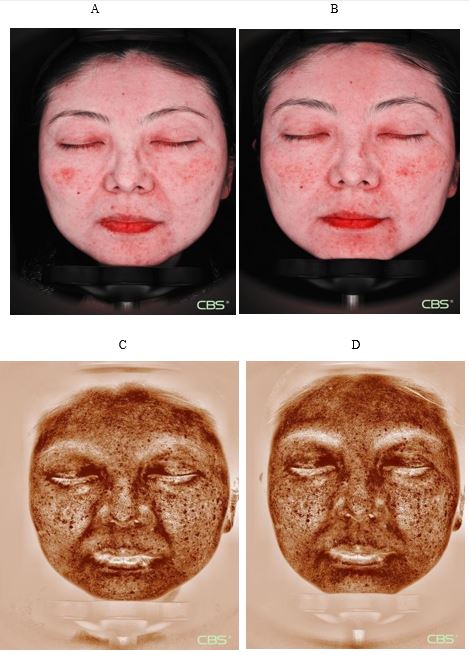
Figure 2: Cloud mirror picture of typical subjects in the collagen peptide vitamin C group
Inflammatory characteristic pattern imaging: Inflammatory reduction. A: 0 day B: 90 day
Brown mode imaging: The pigment is light. C: 0 day D: 90 day
Safety Evaluation
During the test, no adverse events or serious adverse events were observed in all subjects through observation and inquiry. These indicate that the products are safe for use.
Discussion
In this study, the moisture content of facial skin stratum corneum increased significantly after taking two collagen peptide oral liquids for 90 days. The TEWL of facial skin is significantly reduced, indicating that the skin barrier function is repaired and maintained. The test results also demonstrated that the skin retraction time decreased, although not statistically significant, which could indicate an increase in facial skin elasticity. The imaging results showed that after taking the two oral liquids, the superficial and deep pigments of the face, the number of pores, the index values of skin wrinkles, red area, brown area, and porphyrin were all reduced, and the smoothness of the skin was significantly improved. The products have the functions of anti-wrinkle, whitening, improving skin metabolism, and anti-inflammatory effects.
The collagen peptide from the two types of oral liquids, each bottle containing 5000 mg and 6000 mg of collagen peptide, respectively, is derived from tilapia scales. EGCG, known for its strong antioxidant effect, can remove oxygen free radicals, increase the activity of hyaluronic acid synthase, and inhibit hyaluronidase activity. Meanwhile, the addition of high-concentration vitamin C can prevent the lack of vitamin C, a condition in which collagen synthesis cannot form normal fibers.
In recent years, studies have shown that oral collagen peptides can significantly increase skin hydration after 4 weeks. After 8 weeks of oral supplementation of collagen peptide, the density of dermal collagen increased significantly, and the fragments of dermal collagen network decreased significantly. Both effects persisted until 12 weeks after stopping oral administration of collagen peptides. In vitro experiments showed that collagen peptides can induce not only collagen production but also the production of hyaluronic acid, providing an explanation for clinical observations. Seol Hwa Seong et al. reported significant improvement in skin roughness, maximum peak height of wrinkles, and mean maximum height of wrinkles after daily intake of 2 g of collagen peptide. After 12 weeks of collagen supplementation, skin elasticity parameters, including overall elasticity, net elasticity, and bio- elasticity, were significantly improved, and skin hydration and whitening parameters were more significant. The results of the study by Sara Vleminckx et al. showed a significant improvement in dermal density and skin moisture in the collagen group compared to the placebo group after 84 days of 5g of collagen peptide [19].
The results from this study, consistent with previous literature reports, show that collagen peptide products can promote the improvement of facial skin rejuvenation, including increasing the moisture content of the stratum corneum, reducing the loss of skin moisture, and improving skin elasticity. It reduces the number of superficial and deep pigments on the face, the number of pores, skin wrinkles, red area indicators, brown area, and porphyrin values, and improves skin smoothness.
This study also has some limitations. First, there are differences in facial skin conditions between the north and south of China due to temperature and humidity differences. Due to the spatial distribution of the participants in the Beijing area, there are certain geographical limitations, which may limit the universality of the results. Second, as a study of the facial skin status of food supplements, the number of subjects enrolled in each group was relatively small, and the observation time was not sufficient. Therefore, in future studies, we need to further expand the sample size, extend the observation time, conduct regular follow-up, and comprehensively evaluate the effects of collagen peptide.
In conclusion, this study shows that collagen can increase skin moisture, improve skin barrier function, and have anti-wrinkle, whitening, skin metabolism, and anti-inflammatory effects.
CRediT authorship contribution statement
Wenhui Huang: Conceptualization, Methodology, Investigation, Data curation, Validation, Formal analysis, Writing - original draft, Writing - review & editing. Lifang Gao, Huanling Yu, Peiyuan Fan, Le Chen: Conceptualization, Methodology, Investigation. Zupeng Zou, Rui Liu, Zhengnan Liu: Methodology, Software, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research received no specific grants from public, commercial, or not-for-profit funding agencies.