International Journal of Medical Case Reports and Medical Research
OPEN ACCESS | Volume 4 - Issue 1 - 2025
ISSN No: 2994-6905 | Journal DOI: 10.61148/2994-6905/IJMCRMR
Ravi Patel1*, Nnedi Asogwa2, Joanne Ling2, Richa Patel3, Ngowari Pokima2, Varun Sindagi2, Dimitre Stefanov5, Marcin Kowalski4
1Department of Hospital medicine, North Shore University Hospital, Manhasset, NY, United States.
2Department of Internal medicine, Staten Island University Hospital, Staten Island, NY, United States.
3Department of Medicine, OSF Healthcare, Peoria, Illinois, United States.
4Department of Cardiology-Electrophysiology, Staten Island University Hospital, Staten Island, NY, United States.
5Biostatistician, Northwell Health, NY United States.
*Corresponding author: Ravi Patel, Department of Internal medicine, Staten Island University Hospital, Staten Island, NY, United States.
Received: May 20, 2025
Accepted: May 28, 2025
Published: May 30, 2025
Citation: Ravi Patel, Nnedi Asogwa, Joanne Ling, Richa Patel, Ngowari Pokima, Varun Sindagi, Dimitre Stefanov, Marcin Kowalski. (2025) “Assessing the Outcomes of Hospitalized Patients with COVID-19 on Amiodaronr” International Journal of Medical Case Reports and Medical Research, 4(1); DOI: 10.61148/2994-6905/IJMCRMR/169.
Copyright: © 2025 Ravi Patel. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background: In vitro, studies have suggested that amiodarone can inhibit SARS-CoV-2 replication, potentially benefiting COVID-19 patients.
Objective: This study investigates whether patients with a history of atrial fibrillation on amiodarone have improved outcomes compared to those not on amiodarone, focusing on mortality rates, length of hospital stays, and disease severity.
Methods: Using a multiple logistic regression model, we assessed associations between clinical predictors and in-hospital mortality among 616 COVID-19 positive patients with a history of atrial fibrillation. The analysis included comparisons between patients on amiodarone and those not on the medication at admission or discharge. Time-to-event analysis was also performed using the Kaplan-Meier estimator and Cox proportional hazards regression model.
Results: Out of 616 patients, 57 (9.25%) were on amiodarone before admission. In-hospital mortality was observed in 148 (24.03%) patients, with 19.30% in the amiodarone group and 24.31% in the non-amiodarone group. Amiodarone use at admission was not significantly associated with reduced mortality (OR=0.63, 95% CI 0.31-1.28, p=0.20). Cox regression analysis also showed no significant difference in survival (HR=0.601, 95% CI 0.321-1.127, p=0.11). Similarly, amiodarone use did not significantly affect the likelihood of requiring mechanical ventilation (OR=1.366, 95% CI 0.688-2.711, p=0.3732).
Conclusion: Amiodarone use in COVID-19 patients with atrial fibrillation was not significantly associated with improved in-hospital mortality or reduced need for mechanical ventilation. These findings suggest that while amiodarone may have potential in vitro benefits, further clinical trials are needed to establish its efficacy and safety in COVID-19 treatment.
Amiodarone; COVID-19
Introduction:
Amiodarone, a widely used medication for treating arrhythmias such as atrial fibrillation and various heart conditions, has garnered significant attention in the context of COVID-19. It has been investigated as a potential therapeutic option for patients with severe manifestations of the disease. The proposed rationale for exploring amiodarone stems from its immunomodulatory properties and its potential to alleviate inflammation, which could prove beneficial in mitigating the cytokine storm observed in certain COVID-19 patients.
Several in vitro studies have already examined the effects of amiodarone on coronaviruses, including SARS-CoV-2, the virus responsible for COVID-19. These studies have demonstrated either the inhibition of viral replication or a significant reduction in viral load within infected cells when exposed to amiodarone. Such findings suggest the potential benefits of amiodarone in combatting the COVID-19 virus.
Based on these premises, our hypothesis posits that patients with a pre-existing history of atrial fibrillation who were receiving amiodarone at home may experience improved outcomes compared to those not on amiodarone. We aim to compare crucial parameters such as mortality rates, length of hospital stays, and disease severity among COVID-19 positive patients with a history of atrial fibrillation who were either prescribed amiodarone at admission or discharge, in contrast to patients who were not prescribed amiodarone.
By investigating the association between amiodarone use and patient outcomes in the context of COVID-19, this study aims to provide valuable insights into the potential benefits of this medication for individuals with underlying cardiac conditions who contract the disease. These findings may have significant implications for clinical management strategies and inform treatment decisions, ultimately contributing to improved outcomes for COVID-19 patients with atrial fibrillation.
Methods:
We used a multiple logistic regression model to test the associations between a set of clinical predictors and the primary outcome of in-hospital mortality. A similar approach was used for the secondary outcome of Ventilator (Vent) use. Additionally, we analyzed the primary outcome using time to event analysis of in-hospital mortality, considering the Length of Stay (LOS). In this analysis, patients who were discharged alive were censored at the time of their discharge. The Kaplan-Meier estimator of the survival function (long-rank test) and a Cox proportional hazards regression model were used for this analysis. A two-sided p-value<0.05 was considered statistically significant. SAS 9.4 (SAS Institute, Inc, Cary NC) was used for data analysis.
Results:
Sample size: Original data contained 682 visits (hospital admissions) for 616 patients. Among the 616 patients, 560 patients had 1 visit, 46 had 2 and 10 had 3 visits. The median (IQR) age was 79.0 (71.0-86.0) years. There were 336 (54.55%) males in the sample. Demographics and baseline characteristics are reported in the Appendix section of the manuscript. Among the 616 patients, 559/616 (90.75%) did not use Amiodarone, 57/616 (9.25%) used amiodarone before admission and during their entire hospital stay.
In-hospital mortality
We considered the primary outcome of in-hospital mortality [where in-hospital mortality was defined as discharge disposition ='Expired'. Overall, there were 148 (24.03%) patients who died during their hospital stay. There were 11/57 (19.30%) in the amiodarone group, and 137/559 (24.31%) in the group of patients who did not use Amiodarone.
We used a multiple logistic regression model to test for the associations between Amiodarone use and the primary outcome. Adjusted for the confounders [see the table below], we did not find that Amiodarone used at admission was associated with mortality (OR=0.63 95% CI 0.31-1.28, p=0.20), relative to the group of patients for whom Amiodarone was not used.
We found that the history of diabetes (DM) and the history of hypertension were associated with an increased odd of in-hospital mortality [Table 1].
|
Odds Ratio |
|||||
|
Effect |
Value |
95% Confidence Interval |
p-value |
||
|
Age>65 |
Y v N |
1.912 |
0.988 |
3.701 |
0.0542 |
|
Gender |
Male vs Female |
1.005 |
0.685 |
1.474 |
0.9808 |
|
Race_v1 |
Asian vs White |
1.404 |
0.601 |
3.281 |
0.4335 |
|
Race_v2 |
Black vs White |
0.596 |
0.311 |
1.142 |
0.1188 |
|
Race_v3 |
Another vs White |
1.067 |
0.589 |
1.934 |
0.8310 |
|
Race_v4 White |
Unknown vs |
1.702 |
0.337 |
8.605 |
0.5200 |
|
History of DM |
1.528 |
1.016 |
2.297 |
0.0416 |
|
|
History of CAD |
0.589 |
0.326 |
1.065 |
0.0797 |
|
|
History of Heart Failure |
1.100 |
0.712 |
1.700 |
0.6676 |
|
|
History of Hyperlipidemia |
0.948 |
0.594 |
1.512 |
0.8211 |
|
|
History of Hypertension |
1.884 |
1.200 |
2.956 |
0.0059 |
|
|
Amiodarone at Admission |
0.633 |
0.314 |
1.275 |
0.2005 |
|
Table 1: Odds ratio (OR) for the association of the selected predictors and in-hospital mortality
We assessed the association between amiodarone use and in-hospital mortality using time to event analysis. The outcome was the time from admission to death, patients who were discharged alive were censored at the time of their discharge. The Kaplan-Meier estimator of the survival function is presented in the graph below. There we no significant differences between the 2 survival curves (p=0.18, Log-rank test) [Figure 1].
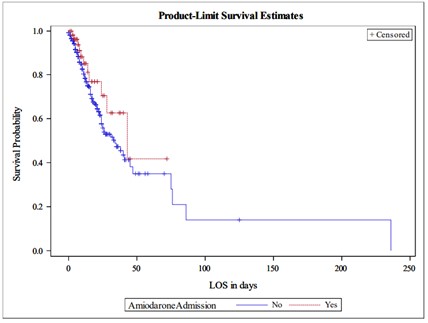
We compared the survival of patients using the Cox proportional hazards regression model. We did not find significant differences between the group of patients who used Amiodarone (HR=0.601, 95% CI 0.321 -1.127, p=0.11) compared to those who did not. We note that the estimated HR<1 along with the 95% CI suggests that our study did not have enough power to demonstrate a protective effect of Amiodarone use. The table with the Hazard ratios (HR) from the Cox proportional hazards model are reported below [Table 2].
Table 2: Hazard Ratio (HR) for the selected predictors with time to in hospital death, based on the Cox proportional hazards model
|
Parameter |
|
Hazard Ratio |
95% Hazard Ratio |
Pr > Chi2 |
|
|
Age>65 |
Y v N |
2.411 |
1.250 |
4.651 |
0.0086 |
|
gender |
Male v Female |
0.920 |
0.658 |
1.286 |
0.6243 |
|
race_v1 |
Asian v White |
1.588 |
0.782 |
3.223 |
0.2008 |
|
race_v2 |
Black v White |
0.649 |
0.351 |
1.203 |
0.1700 |
|
race_v3 |
Other v White |
1.107 |
0.661 |
1.852 |
0.6999 |
|
race_v4 |
Unknown v White |
2.380 |
0.555 |
10.202 |
0.2429 |
|
History of DM |
Y v N |
1.283 |
0.901 |
1.826 |
0.1667 |
|
History of CAD |
Y v N |
0.617 |
0.363 |
1.048 |
0.0738 |
|
History of Heart failure |
Y v N |
1.036 |
0.705 |
1.524 |
0.8554 |
|
History of Hyperlipidemia |
Y v N |
0.878 |
0.586 |
1.315 |
0.5271 |
|
History of Hypertension |
Y v N |
1.625 |
1.107 |
2.385 |
0.0132 |
|
Amiodarone at Admission |
Y v N |
0.601 |
0.321 |
1.127 |
0.1125 |
Ventilator use
We considered the use of mechanical ventilator as a secondary outcome. Overall, ventilator was used in 92/559 (16.46%) patients in the non-amiodarone group, and 12/57 (21.05%) in the Amiodarone group. We used a multiple logistic regression model to investigate the association between amiodarone use and Vent use. Adjusted for the other risk factors, Amiodarone use was not associated with ventilator use during hospitalization (OR=1.366, 95% CI 0.688-2.711, p=0.3732) [Table 3].
Table 3: Odds Ratio for the association of the selected predictors and the use of Ventilator
|
Odds Ratio |
|||||
|
Effect |
Value |
95% Wald Confidence Limits |
p-value |
||
|
Age>65 |
1 vs 0 |
0.849 |
0.463 |
1.557 |
0.5969 |
|
Gender Female |
Male vs |
1.832 |
1.171 |
2.867 |
0.0080 |
|
Race_v1 White |
Asian vs |
1.505 |
0.589 |
3.844 |
0.3929 |
|
Race_v2 White |
Black vs |
0.670 |
0.318 |
1.412 |
0.2926 |
|
Race_v3 White |
Other vs |
1.707 |
0.931 |
3.129 |
0.0839 |
|
Race_v4 White |
Unknown vs |
1.646 |
0.327 |
8.292 |
0.5457 |
|
History of DM |
1.268 |
0.805 |
1.997 |
0.3052 |
|
|
History of CAD |
0.539 |
0.266 |
1.093 |
0.0867 |
|
|
History of heart failure |
0.888 |
0.544 |
1.448 |
0.6331 |
|
|
History of hyperlipidemia |
1.090 |
0.651 |
1.822 |
0.7438 |
|
|
History of hypertension |
1.105 |
0.660 |
1.850 |
0.7035 |
|
|
Amiodarone at admission |
1.366 |
0.688 |
2.711 |
0.3732 |
|
Discussion:
COVID Pathophysiology
Coronavirus disease 2019 (COVID-19) is a respiratory illness caused by the novel coronavirus SARS-CoV-2. The transmission of COVID-19 occurs primarily through respiratory droplets that are generated when an infected person coughs, sneezes, talks, or breathes [1]. The pathophysiology of COVID-19 involves virus attaching to host cells, replicating, causing inflammation and tissue damage, and triggering a dysregulated immune response. These processes can lead to respiratory failure, cardiovascular damage, and multi-organization failure, among other complications [2]. Understanding the pathophysiology of COVID-19 is critical in developing effective treatments and preventative measures for this disease.
The structure of the coronavirus is key to understanding how it infects cells and causes disease. The virus is an enveloped, positive-sense single-stranded RNA virus [3]. The outer envelope of the virus is a lipid membrane derived from the host cell, which contains three main structural proteins: the spike (S) protein, the membrane (M) protein, and the envelope (E) protein [4].
These structural proteins play a crucial role in the viral entry, binding, assembly, release, and ultimately replication [3]. Understanding the structure of the coronavirus is important for the development of vaccines and antiviral drugs, as it allows researchers to identify targets for intervention. The spike protein, for example, is a prime target for vaccine development, as antibodies that target this protein can prevent the virus from entering human cells [5]. Similarly, antiviral drugs that target the viral enzymes involved in replication or assembly of the virus can help to prevent or treat COVID-19.
COVID Epidemiology:
The global impact of COVID-19 has been profound, with over 754 million confirmed cases and
6.8 million deaths reported worldwide by February 2023 [6]. The disease affects individuals of all ages, posing a particularly high risk to older adults and those with preexisting conditions such as diabetes, heart disease, and respiratory illnesses, who are more susceptible to severe outcomes [7]. The clinical manifestations of COVID-19 range from mild or asymptomatic cases to severe conditions characterized by respiratory distress, pneumonia, and multi-organ failure [8]. A critical challenge in managing the spread of COVID-19 is its incubation period, ranging from 2 to 14 days, during which infected individuals can transmit the virus without showing symptoms, complicating efforts to contain the virus [7][8].
Amiodarone:
Amiodarone, a cornerstone in the treatment of various arrhythmic disorders, operates through a multifaceted mechanism of action. It primarily modulates the ion channels within the cardiac cells, significantly affecting the cardiac action potential by blocking potassium and calcium channels which are crucial for cell repolarization [9]. Moreover, amiodarone exhibits anti-alpha- adrenergic activity and antianginal properties, contributing to its broad therapeutic effects [9].
Unlike some anti-arrhythmic drugs, amiodarone maintains a neutral stance on myocardial contractility and does not compromise the oxygen supply to cardiac tissues, making it a preferred option for managing complex arrhythmias [10][11].
Despite its therapeutic benefits, amiodarone's use is associated with a spectrum of potential side effects. Common adverse reactions include gastrointestinal disturbances like nausea and vomiting, neurological effects such as tremors and ataxia, and dermatological changes. More concerning are its implications on thyroid function, leading to hypothyroidism, and the risks of pulmonary and liver toxicity, which present significant clinical challenges [12]. The incidence of pulmonary toxicity, one of the most severe side effects, ranges from 2% to 17%, whereas liver toxicity appears in less than 3% of the treated population [13]. Additionally, amiodarone has been linked to symptomatic bradycardia, underscoring the need for meticulous patient monitoring [13].
The therapeutic application of amiodarone spans across various age groups, tailored to address specific arrhythmic conditions. In adults, it is frequently prescribed for managing life-threatening ventricular tachycardia and fibrillation, as well as atrial fibrillation, demonstrating efficacy in restoring normal sinus rhythm and improving survival rates [13]. Pediatric use, although less common, focuses on treating supraventricular tachycardia (SVT), with guidelines suggesting its use in refractory or recurrent cases [13]. However, its prescription in elderly patients warrants caution due to the heightened risk of adverse reactions and the drug's interaction with existing comorbidities, necessitating a balanced assessment of benefits against potential risks [13].
A-Fib Definition and Epidemiology
Atrial fibrillation, characterized by irregular and often rapid heart rate resulting from asynchronous atrial contractions, stands as the most prevalent form of arrhythmia globally [14]. Its incidence escalates with age, markedly affecting older populations and those with underlying cardiovascular conditions. A-fib contributes to significant morbidity, elevating the risk of stroke, heart failure, and other cardiovascular complications. Epidemiological data reveal a gender disparity in a-fib prevalence, with higher incidence rates observed in men compared to women and underscore the influence of modifiable risk factors such as hypertension, obesity, and lifestyle choices on disease onset [14].
Amiodarone in A-Fib and In Vitro Activity Against COVID-19:
Amiodarone's efficacy in managing atrial fibrillation is well-documented, outperforming other anti-arrhythmic agents in maintaining sinus rhythm and offering a viable treatment pathway for patients with this condition [15][16]. Beyond its conventional use, emerging in vitro studies have explored amiodarone's potential utility against COVID-19. Amiodarone has shown promise as a potential therapeutic agent against the COVID-19 virus, as evidenced by several in vitro studies. One study demonstrated its efficacy in inhibiting the replication of SARS-CoV-2 [17]. This study revealed significant inhibition of viral RNA replication and protein synthesis in Vero cells infected with the virus. Similarly, another study found that amiodarone effectively reduced the viral load in infected Vero E6 cells [18]. These findings suggest that amiodarone holds promise as a treatment option for COVID-19.
Moreover, additional research provided further support for the antiviral activity of amiodarone, showing its ability to decrease the expression of ACE2 receptors, thereby impeding viral entry into host cells [19]. Additionally, it was demonstrated that amiodarone disrupts the endocytic pathway crucial for SARS-CoV-2 infectivity [20].
In conclusion, these in vitro studies underscore the potential therapeutic benefits of amiodarone against coronavirus infections. However, further clinical trials are necessary to assess its efficacy and safety in COVID-19 patients.
Statements and Declarations:
This manuscript is an original submission
The contents of this manuscript have not been published or submitted for publication elsewhere. All authors read and approved the final manuscript for submission.
Availability of data and material: The data and materials associated with this manuscript are available upon request. Researchers, scholars, or individuals interested in accessing specific datasets, laboratory protocols, or any other materials related to the study can contact the corresponding author for further information
Competing interests: There are no competing interests to declare.
Funding: No funding was received for preparing this manuscript. The authors have no financial or proprietary interest in any material discussed in this article.
Acknowledgments: Not applicable
Institutional Review Board Statement: Not applicable.
Appendix:
A. Demographics
Table A1: Age of all patients included in this study at Hospital Admission
|
Total |
Minimum |
25th Percentile |
50th Percentile |
75th Percentile |
Maximum |
Mean |
Standard Deviation |
|
616 |
21.0 |
71.0 |
79.0 |
86.0 |
102.0 |
77.6 |
11.6 |
Table A2: Gender of all patients included in this study
|
Gender |
Frequency |
Percentage |
Cumulative frequency |
Cumulative Percentage |
|
Female |
280 |
45.45 |
280 |
45.45 |
|
Male |
336 |
54.55 |
616 |
100.00 |
Table A3: Gender frequency and percentage of patients included in this study who were on amiodarone and those who were not on amiodarone
|
Frequency Percentage |
No |
Yes |
Total |
|
Female |
258 46.15 |
22 38.60 |
280 |
|
Male |
301 53.85 |
35 61.40 |
336 |
|
Total |
559 |
57 |
616 |
Table A4: Race of all patients included in this study who were on amiodarone and those who were not on amiodarone
|
Frequency Percentage |
No |
Yes |
Total |
|
Asian |
25 4.47 |
3 5.26 |
28 |
|
Black |
74 13.24 |
3 5.26 |
77 |
|
White |
383 68.52 |
48 84.21 |
431 |
|
Other |
69 12.34 |
3 5.26 |
72 |
|
Unknown |
8 1.43 |
0 0.00 |
8 |
|
Total |
559 |
57 |
616 |
Table A4: History of Diabetes in all patients included in this study who were on amiodarone and those who were not on amiodarone
|
Frequency Percentage |
No |
Yes |
Total |
|
No history of Diabetes |
294 52.59 |
22 8.60 |
316 |
|
Had history of Diabetes |
265 47.41 |
35 61.40 |
300 |
|
Total |
559 |
57 |
616 |
Table A5: History of Coronary Artery Disease (CAD) in all patients included in this study who were on amiodarone and those who were not on amiodarone
|
Frequency Percentage |
No |
Yes |
Total |
|
No history of CAD |
487 87.12 |
45 78.95 |
532 |
|
Had history of CAD |
72 12.88 |
12 21.05 |
84 |
|
Total |
559 |
57 |
616 |
Table A6: History of Heart Failure (HF) in all patients included in this study who were on amiodarone and those who were not on amiodarone
|
Frequency Percentage |
No |
Yes |
Total |
|
No history of CAD |
487 87.12 |
45 78.95 |
532 |
|
Had history of CAD |
72 12.88 |
12 21.05 |
84 |
|
Total |
559 |
57 |
616 |
Table A7: History of Hyperlipidemia (HLD) in all patients included in this study who were on amiodarone and those who were not on amiodarone
|
Frequency Percentage |
No |
Yes |
Total |
|
No history of HLD |
166 29.70 |
9 15.79 |
175 |
|
Had history of HLD |
393 70.30 |
48 84.21 |
441 |
|
Total |
559 |
57 |
616 |
Table A8: History of Hypertension (HTN) in all patients included in this study who were on amiodarone and those who were not on amiodarone
|
Frequency Percentage |
No |
Yes |
Total |
|
No history of HTN |
350 62.61 |
27 47.37 |
377 |
|
Had history of HTN |
209 37.39 |
30 52.63 |
239 |
|
Total |
559 |
57 |
616 |