International Journal of Medical Case Reports and Medical Research
OPEN ACCESS | Volume 4 - Issue 1 - 2025
ISSN No: 2994-6905 | Journal DOI: 10.61148/2994-6905/IJMCRMR
Sarah Noor1, Ahmed Maseh Haidary2*, Ahmed Nasir Hanifi3, Ahmad Shekib Zahier4, Maryam Ahmad2, Sahar Noor5, Muhibullah Rahmani2, Ramin Saadaat2 and Esmatullah Esmat2.
1Department of Haemato-oncology, Ali Abad Hospital, Kabul, Afghanistan.
2Department of Pathology and Clinical Laboratory, French medical institute for mothers and children (FMIC), Kabul, Afghanistan.
3Department of Pathology, Central Public Health Laboratory, Ministry of Public Health, Kabul, Afghanistan.
4Department of Haemato-oncology, Amiri Medical Complex, Kabul, Afghanistan.
5Department of Paediatrics, French Medical Institute for Mothers and Children, Kabul, Afghanistan.
*Corresponding Author: Ahmed Maseh Haidary, Department of Pathology and Clinical Laboratory, French medical institute for mothers and children (FMIC), Kabul, Afghanistan.
Received Date: August 01, 2024
Accepted Date: August 12, 2024
Published Date: September 15, 2024
Citation: Sarah Noor, Ahmed Maseh Haidary, Ahmed Nasir Hanifi, Ahmad Shekib Zahier, Maryam Ahmad.et.al. (2024) “Bone Marrow Metastasis by An Occult Prostatic Adenocarcinoma Mimicking Essential Thrombocythaemia.”, International Journal of Medical Case Reports and Medical Research, 3(3); DOI: 10.61148/2994-6905/IJMCRMR/051.
Copyright: © 2024. Ahmed Maseh Haidary. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background: Bone marrow is infiltrated by carcinomas and sarcomas as a terminal event. Marrow involvement usually presents with peripheral blood cytopaenia due to marrow failure. It is least likely to encounter a patient with marrow infiltration mimicking myeloproliferative neoplasms. Here, we present a case of metastatic adenocarcinoma of prostate that clinically mimicked essential thrombocythaemia
Case Presentation: A 63-Year-Old man with underlying stroke, was noted to have increase in peripheral blood platelet count during routine laboratory workup. Thrombocytosis was noted to be persistent for three months while all infectious and inflammatory markers were negative. Bone marrow analysis revealed infiltration by an occult prostate adenocarcinoma.
Conclusion: Bone marrow is a complex organ and any infiltration would affect the internal micro-environment, presenting with a wide spectrum of clinical presentation. To the best of our knowledge, this was the first case of prostate cancer with clinical presentation mimicking essential thrombocythaemia.
Key Clinical Message:
Our patient presented with thrombocytosis that persisted during the span of 3 months of follow up.
Bone marrow analysis revealed an underlying prostate adenocarcinoma, rather than myeloproliferative disorder, as the causes of thrombocytosis.
Background:
Metastatic involvement of the bone marrow usually occurs by solid organ tumors that metastasize via the blood stream, such as adenocarcinomas of the prostate, breast and lung in adults, as well as neuroblastoma, rhabdomyosarcoma, Ewing’s sarcoma and retinoblastoma in paediatric age group [1,2]. The bone marrow parenchyma as well as the stromal micro-envirnomen responds variably to such metastatic infiltrations. Therefore, metastatic infiltrations of the bone marrow can have variable clinical presentations with some being asymptomatic while others presenting with wide spectrum of clinical symptoms and haematological manifestatons [3].
Prostatic cancer with bone marrow involvement has even been reported to present as thrombotic thrombocytopenic purpura [4]. Secondary infiltration of the bone marrow by any primary neoplastic process has significant impact on both management strategy as well as prognosis [5]. Therefore, bone marrow analysis is an important diagnostic modalities that can be utilized for staging of solid organ tumours and lymphomasb [6].
Here we present an interesting case of clinically overlooked prostate cancer in a chronically debilitated old man, who was initially worked-up with impression of essential thrombocythaemia, but bone marrow analysis was conclusive of bone marrow infiltration by prostate adenocarcinoma.
Case Presentation:
A 63-Year-Old man, with underlying debilitating ischemic stroke for the last 3 years, was noted to have mild normochromic normocytic anaemia, mild leukocytosis, unremarkable differential leukocyte counts with elevated platelet counts on multiple occasions with the highest recorded value being 1,122 × 103 platelets/ microliters, shown in table 1. The peripheral blood film demonstrated true thrombocytosis with leucoerythroblastic anemia. Thrombocytosis was persistent during 3 months of follow-up. On examination, the patient had no organomegaly, no lymphadenopathy and no constitutional symptoms. The iron stores were normal, JAK2, MPL1 and Calreticulin mutations were negative. Similarly, antinuclear antibody (ANA) was non-reactive. With an impression to rule-out essential thrombocythaemia (ET), a bone marrow analysis was performed. The bone marrow aspirate was hemodiluted with presence of clumps of cohesive large pleomorphic tumor cells, with large cell size, high nucleo-cytoplasmic ratio, irregular nuclear outlines, hyperchromatic nuclei and variable amount of basophilic cytoplasm, as shown in figure 1. The trephine biopsy showed hyper-cellular marrow with diffuse infiltration by neoplastic cells, resulting in loss of normal marrow architecture as shown in figure 2a. Normal haemopoietic progenitors, including megakaryocytes were almost completely displaced. Immuno-histochemical (IHC) staining revealed that the neoplastic cells were positive for Pan cytokeratin (CK) not shown in the images, while both CK7 and CK20 were negative as shown in figures 2 b and 2 c respectively. Similarly, IHC for Prostate specific antigen (PSA) was strongly positive, as shown in figure 2 d. Thus, the bone marrow analysis was concluded with the impression of metastatic bone marrow involvement by adenocarcinoma of the prostate. Subsequently, Transurethral Resection of Prostate (TURP) was performed and tissue sent for histopathology examination was constituted by multiple gray-white prostatic chips tissue, measuring 4 x 3cm in total aggregations. The haematoxylin & eosin-stained tissue sections revealed sheets of neoplastic cells, demonstrating fused, cribriform and few single and separate glandular arrangement. The neoplastic cells were large and pleomorphic with enlarged hyperchromatic nuclei and prominent nucleoli, consistent with the diagnosis of prostate adenocarcinoma, with Gleason score 4 + 3 = 7/10 and grade group of 3, based on Gleason scoring (Fig.3a & b).
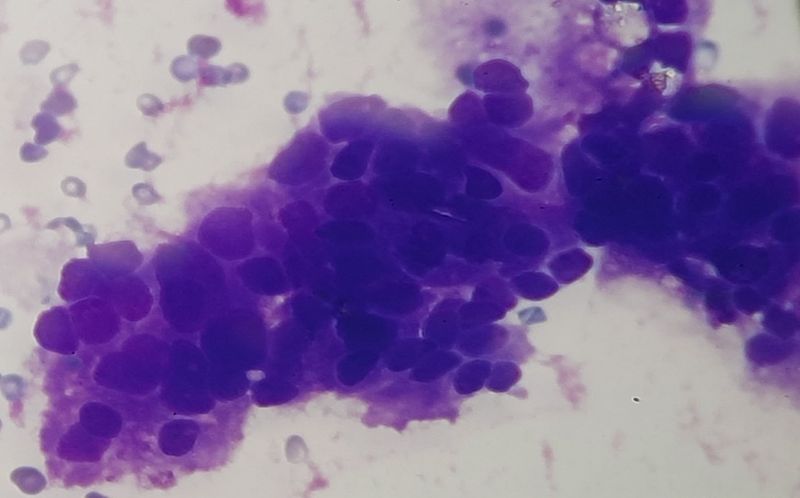 Figure 1: Haemodiluted bone marrow aspirate smear slides showing cohesive clusters of neoplastic cells.
Figure 1: Haemodiluted bone marrow aspirate smear slides showing cohesive clusters of neoplastic cells.
|
|
11th November 2019 |
20th December 2019 |
18th March 2020 |
|
Hemoglobin (grams/decilitre) |
11.3 |
10.1 |
12.0 |
|
Leukocyte count (x 103/ microliter) |
13000 |
11000 |
15000 |
|
Platelet count (x 103/ microliter) |
8658 |
10325 |
1120 |
|
Peripheral blood film |
- |
- |
Leucoerythroblastic reaction |
Table 1: Haematology parameters during follow up.

Figure 2: (a): Haematoxylin and Eosin-stained sections of the bone marrow showing diffuse sheaths of neoplastic cells, occupying the marrow spaces, (b): CK7 IHC showing negative reaction, (c): CD20 IHC showing negative reaction and (d): PSA IHC showing strong cytoplasmic positivity in the infiltrating neoplastic cells.
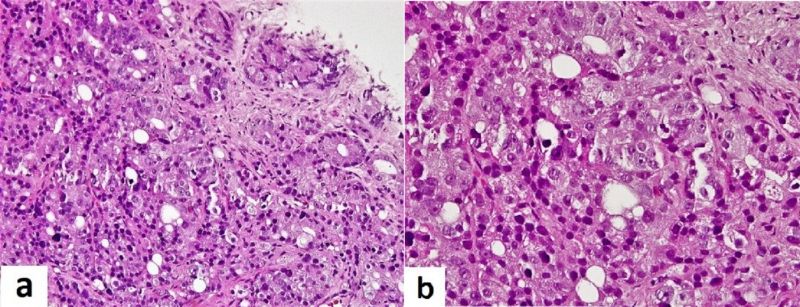 Figure 3: (a): showing predominantly poorly formed/fused/cribriform glands and few well-formed and separately present neoplastic glands. (b): showed higher magnification of the neoplastic glands with enlarged hyperchromatic nuclei and prominent nucleoli.
Figure 3: (a): showing predominantly poorly formed/fused/cribriform glands and few well-formed and separately present neoplastic glands. (b): showed higher magnification of the neoplastic glands with enlarged hyperchromatic nuclei and prominent nucleoli.
Discussion:
Metastatic bone marrow involvement by a primary solid organ tumour occurs as a terminal event. Detection of an occult primary non-haematologic malignancy with bone marrow involvement during workup for another primary haematologic malignancy is very rare [7].
Most of the bone marrow metastatic diseases results in reactive fibrosis and thus peripheral blood cytopaenia [7]. This is due to the interplay of marrow microarchitecture and the cytokines released by the metastatic tumour [8]. In some cases, the bone marrow trephine biopsy consists in part or as a whole, of necrotic tissue [7]. Therefore, when necrotic tissue is encountered in a bone marrow biopsy, then secondary tumour metastasis should be one of the differential diagnosis, thus further workup, including repeated bone marrow biopsy should be considered [9].
Whenever marrow infiltration by a suspected non-haematological tumour is encountered, the patterns of cytokeratin (CK) immunohistochemical (IHC) staining are particularly helpful for the detection of the primary source [10]. In such cases a series of IHC stains are required for identification of the primary tumour [10]. The diagnostic workup would also require thorough history taking, complete physical examination and radiological correlation with appropriate pathological sampling and investigation [10].
In our patient the clinical presentation including initial laboratory workup were suggestive of myeloproliferative neoplasm, likely essential thrombocythaemia, but bone marrow analysis revealed the infiltration by prostatic adenocarcinoma, which was subsequently confirmed by performing prostatic tissue excision biopsy. In many of the instances the patient would present with varying degrees of peripheral blood cytopaenia [11]. In our patient, the possible mechanism for the tumour infiltration induced thrombocytosis could be explained by the milieu of cytokines released by the tumour cells as well as response of the reactive stroma of the infiltrated bone marrow [8]. Many authorities have demonstrated the role of bone marrow stromal cells, parenchymal haemopoietic cells, the infiltrating tumour cells and the released cytokines, especially bone morphogenic protein 4, stem cell factor, IL-3, IL-6, and IL-9 in resulting in bone marrow megakaryocytic proliferation with resultant reactive thrombocytosis [12].
Our patient did not have any of the morphological features suggestive of myeloproliferative disorders; especially considering the megakaryocytic morphology that would suggest essential thrombocythemia [13]. It is always important to rule out secondary causes of thrombocytosis in a patient with unexplained and persistent thrombocytosis.
Conclusion:
Effect of the wide spectrum of cytokines released by tumor cells, can significantly affect the behavior of bone marrow parenchyma as well as stroma, resulting in wide spectrum of unpredictable clinical presentation. To our knowledge this was the first case of prostate adenocarcinoma with bone marrow infiltration that presented with thrombocytosis, mimicking ET.
Abbreviations:
JAK2: Janus activated kinase-2
ANA: anti-nuclear antibodies
ET: essential thrombocythemia
CK: cytokeratin
PSA: prostate specific antigen
IHC: Immunohistochemical
BMT: bone marrow trephine biopsy.
Declarations:
Authors contributions: AMH, SRN, ASZ and RS conceived the idea. AMH, ANH, MR and EE were the major contributor to the writing of the manuscript. MA traced the laboratory data. MA performed the pathological processing. EE, AMH and RS diagnosed the case. SHN, SRN, AMH, ASZ, ANH, RS were the major contributors for critically revising the manuscript for important intellectual content. SRN and AMH have given expert opinion and final approval of the version to be published. All authors read and approved the final manuscript.
Acknowledgement: None.
Funding: Authors received no funding for the writing.
Availability of data and material: All the generated data are included in this article.
Ethical approval and consent to participate: Ethical approval was acquired from the hospital’s ethical review committee. Informed consent was obtained from the patient for participation in the current case report. The Abstract of the case was presented in the 13th Annual scientific meeting of the FMIC, Held in November 2023, Kabul Afghanistan; "https://www.fmic.org.af/AboutUs/Documents/Abstract%20Book-2023.pdf".
Consent for publication: Written informed consent was obtained from patient for publication of this case report and all the accompanying figures. A copy of the written consent shall be availed to the Editor-in-Chief of this journal.
Competing interests: The Authors declare that they have no competing interests.