International Journal of Interventional Radiology and Imaging
OPEN ACCESS | Volume 3 - Issue 1 - 2025
ISSN No: 3065-6702 | Journal DOI: 10.61148/3065-6702/IJIRI
Dr Roshan Valentine, MBBS MD DNB MNAMS FVIR EBIR 1*, Dr Krishnendhu M S MBBS DNB FVIR EBIR2, Dr Trupthi Das MBBS MD DNB FVIR 3, Dr Uthappa M C MBBS FRCS FRCR CCST 4
1Associate consultant, Department of Interventional Radiology, Manipal Hospital, Bengaluru, India.
2Specialist, Department of Interventional Radiology, Manipal Hospital, Bengaluru, India.
3Fellow, Department of Interventional Radiology, Manipal Hospital, Bengaluru, India.
4Consultant – Head of Interventional Radiology, Gleneagles Hospitals, Bengaluru, India.
*Corresponding author: Roshan Valentine, Associate consultant, Department of Interventional Radiology, Manipal Hospital, Bengaluru, India.
Received: April 25, 2025
Accepted: May 01, 2025
Published: May 05, 2025
Citation: Roshan Valentine, Krishnendhu,M S Trupthi Das, Uthappa M C., (2025) “Unusual Cause of Encephalopathy in A Non-Cirrhotic Adult: Case Report and Review of Literature.” International Journal of Interventional Radiology and Imaging, 3(1); DOI: 10.61148/3065-6702/IJIRI/046.
Copyright: © 2025 Roshan Valentine. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Portosystemic shunts are abnormal connections between the portal vein and the systemic veins bypassing the liver. Anatomically, they are divided into intrahepatic and extrahepatic shunts. Mr. Abernathy first described extrahepatic shunts in 1793, who reported an absent portal vein and congenital mesenterico-caval shunt on a 10-month-old child. (1,2) This causes all the nitrogenous and impure substances in the blood to enter the systemic circulation. This is commonly seen in patients with liver cirrhosis secondary to portal hypertension. However, we present a case report of a non-cirrhotic intrahepatic portosystemic shunt with encephalopathy referred to as type B encephalopathy in a 72-year-old female with acute onset confusion and worsening cognitive impairment.
encephalopathy; abernathy malformation; portosystemic shunt; portal hypertension; liver cirrhosis
CASE REPORT:
A 72-year-old female with complaints of fluctuating levels of altered sensorium, drowsiness, rigidity, and tremors on evaluation was diagnosed to have metabolic encephalopathy due to elevated levels of serum ammonia (serum ammonia -153 micro/dL), and EEG showed diffuse slowing of background with triphasic wave suggestive of metabolic encephalopathy. She was initially managed by a medical gastro team with a restricted protein diet, lactulose, rifamixin, and hepamerz and a daily enema was given for stool clearance.
Given no clinical improvement and persistent higher levels of serum ammonia of 161 microgram/dL, The reference was given to the IR team for further management. The patient had a background history of adenocarcinoma vulva with localized lymph nodal metastasis post-wide local excision, and she was on radiotherapy and chemotherapy for the same. Suspicion for a portosystemic shunt on PET CT was confirmed with CECT abdomen and pelvis.
A multi-disciplinary meeting involving the neurologist, gastroenterologist, medical oncologist, and interventional radiologist was held, and an endovascular embolization option was decided.
Under general anesthesia and ultrasound guidance, the right posterior branch of the portal vein was punctured using a Neff set(21G), and access was secured with a 5-fr vascular sheath. The main portal vein was cannulated with a 5FR hydrophilic cobra catheter, and a portogram showed an early draining hepatic vein in concordance with the CT.
Selective cannulation of the segment VIII portal vein was done using a 0.035-inch hydrophilic glide wire (Terumo) followed by the catheter. Selective cannulation and venogram demonstrated the intrahepatic portosystemic shunt. However, given the increased tortuosity and increased propensity for the hydrophilic wire to get flipped off from the site, a 2.7Fr Progreat microcatheter was used to cannulate the portal vein and to provide additional support. A 4 x 14mm micro nester coil was used to occlude the shunt, and post-embolization venography demonstrated absent flow through the shunt. The patient had a dramatic improvement in her cognition and speech the day after the procedure, with a reduction of serum ammonia level to the normal range.
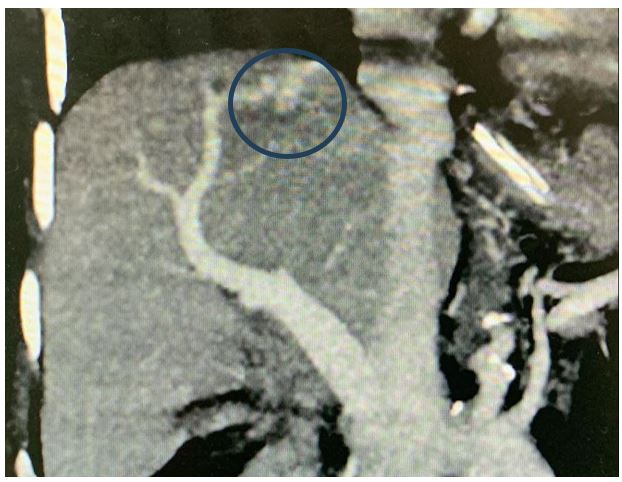
Figure 1: Coronal MIP showing the Porto venous shunt (blue circle)
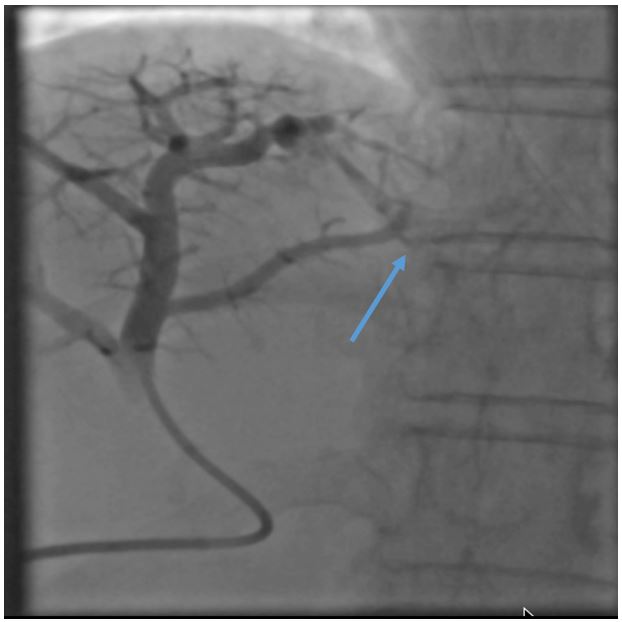
Figure 2: Portogram showing early draining hepatic venous channel (Blue arrow).
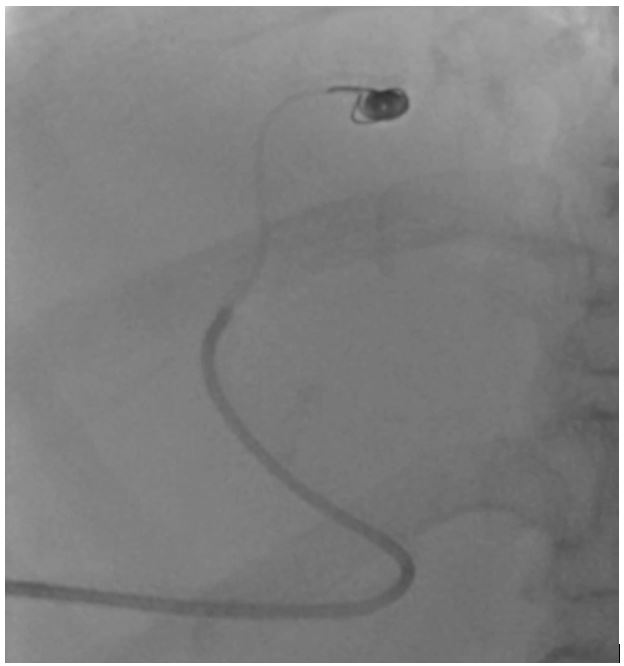
Figure 3: Selective cannulation and coil embolisation of the portosystemic shunt.
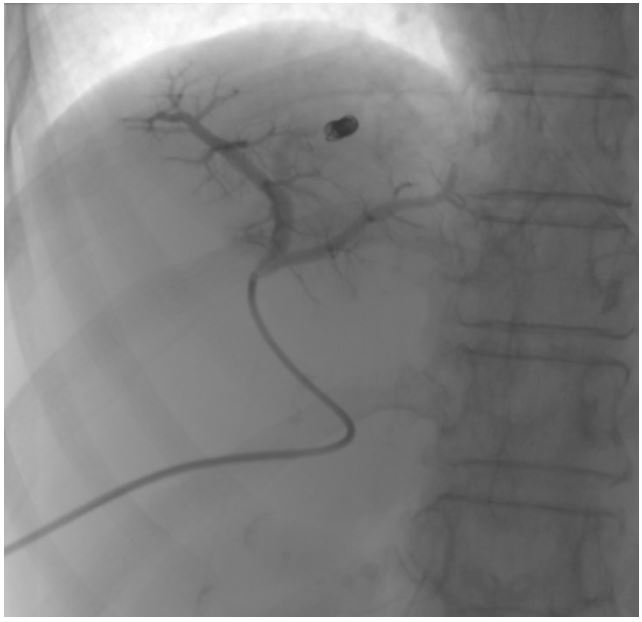
Figure 4: Post-coil embolisation portogram showing non-opacification of the early draining vein.
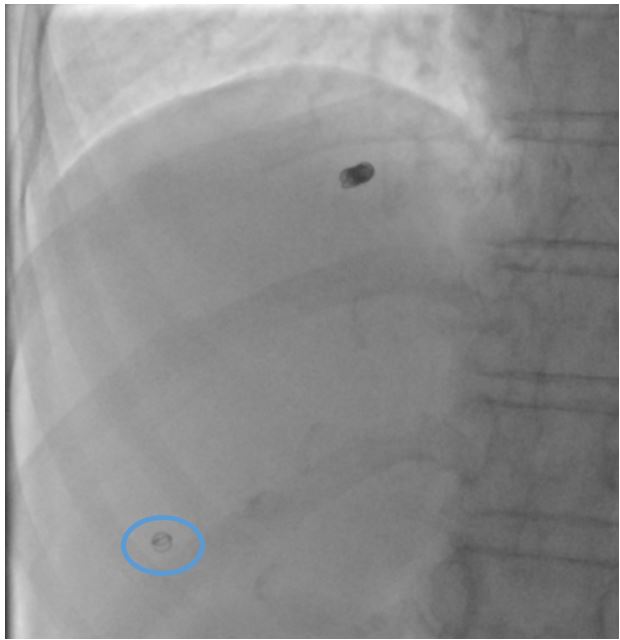
Figure 5: Post-embolisation image showing percutaneous tract embolisation with a micro coil (Blue circle).
Discussion:
Intrahepatic portosystemic shunts are a relatively rare entity that can be either asymptomatic or can result in debilitating cognitive decline. (3) However, earlier diagnosis and management can often be completely curative. (4) While symptomatic, they often get misdiagnosed for a psychiatric cause, which can result in further delays in initiating proper treatment and adding to the worry of the kin. (5)(6,7). The frequency of reporting such cases is increasing due to the advancement of imaging techniques and more awareness among clinicians. (5) These patients can present with encephalopathy, pulmonary hypertension, hepatopulmonary syndrome, hypoglycemia, or bleeding with relatively normal liver function and no splenomegaly. (8)
The origin of intrahepatic PSS is less understood. However, two major theories have been proposed: the congenital origin theory and the acquired theory. Former, as the name suggests, occurs due to the persistent communication between the hepatic and portal venous systems due to aberrant embryonal development. The acquired theory is of the suggestion that it occurs secondary to portal hypertension, trauma, and secondary to liver biopsy. (4). The intrahepatic portal–systemic venous shunt is thought to represent persistent communication between cranial and caudal hepatic sinusoids formed by vitelline veins and umbilical veins. (4)
However, acute manifestation of these shunts in older people may be attributed to the fragility of the blood vessels and hemodynamic changes with aging. ((9) For better understanding and treatment planning, intrahepatic PSS have been classified into two types based on the shunt morphology: shunt consisting of intrahepatic portal venous–hepatic venous pathway Intrahepatic portal venous–perihepatic venous pathway that consists of inferior phrenic veins, adrenal veins, and paraumbilical veins. (11)
Park et al classified them into four types, which were further modified by Gallego et al. (12,13):
Type 1: a single large vessel of constant diameter connecting the right portal vein to the inferior vena cava.
type 2: localized, peripheral shunt with one or more communications between the hepatic and portal vein in a single hepatic segment
type 3: the aneurysmal connection between peripheral portal and hepatic vein segments
type 4: multiple communications between the peripheral portal and hepatic veins within both hepatic lobes
type 5: persistent ductus venosus
These patients can easily be diagnosed with increased serum ammonia levels with no signs of liver dysfunction or other causes attributed to hyperammonemia like hematological malignancies, infection with urease-producing organisms, drugs like valproate, carbamazepine, salicylates, and sulfadiazine., urea cycle defects unmasked by increased muscle catabolism due to seizures and starvation, increased protein load delivered in total parenteral nutrition, gastrointestinal bleeding, hemodialysis and infections. (14–17). When psychoneurological symptoms are suggestive of hepatic encephalopathy but objective and subjective symptoms or abnormal values of liver function tests are not sufficiently indicative of liver cirrhosis, portal-systemic encephalopathy should be suspected. (5)
Conservative therapy (restriction of protein, ingestion of lactulose, oral administration of nonabsorbable antibiotics, sodium benzoate, and phenylacetate) (5), transcatheter embolization, and surgery have been used for the treatment of intrahepatic portosystemic venous shunt. (4)
According to Kudo et al., therapeutic intervention Is warranted when the patient has hepatic encephalopathy or with venous shunt greater than 60%. (18) According to the degree of invasiveness, there are three types of endovascular methods for the treatment of PSS, namely Retrograde transcaval obliteration, percutaneous transhepatic obliteration, and trans ileocolic obliteration. While the first two methods can be performed under local anesthesia, the latter requires epidural or anesthetic support. (4) Various occlusive devices can be used like coils(pushable and detachable), vascular plugs, n butyl cyanoacrylate.(19,20) The percutaneous transhepatic approach is advantageous in accessing the contralateral distribution of the shunt as it provides better catheter control. However, these are not free from complications as, according to a study, 16% of their patients treated with this method developed procedure-related complications. (21,22) Post-procedure, trans parenchymal tract embolization with coils and glue is required to prevent bleeding from the access site. Embolizations with gel foam are often insufficient as post-shunt obliteration; the increased pressure in the portal system can dislodge the hemostasis. (23) Trans ileocolic obliteration has the easiest access and control of the catheters due to its antegrade approach while being more invasive. However, it requires an abdominal incision to access the ileocolic veins and the portal vein. (4) Thus, earlier diagnosis and treatment of this unusual cause of encephalopathy in a non-cirrhotic patient can be rewarding to the patient, the treating physician, and the interventional radiologist. Understanding the morphology of the shunt and choosing the ideal access is an important part of the treatment.
Declaration of conflicting interests:
The authors declared no potential conflicts of interest concerning the research, authorship, and/or publication of this article.
FUNDING
The authors received no financial support for the research,
authorship, and/or publication of this article.
Acknowledgement: nil