International Journal of Integrative and Complementary Medicine
OPEN ACCESS | Volume 2 - Issue 1 - 2026
ISSN No: 3070-4146 | Journal DOI: 10.61148/3070-4146/IJICM
Rehan Haider1*, Zameer Ahmed2, Hina Abbas3, Shabana Naz Shah4, Geetha Kumari Das5, Sambreen Zameer6
1Head of Marketing and Sales, Riggs Pharmaceuticals, Karachi; Department of Pharmacy, University of Karachi, Pakistan.
2Assistant Professor, Department of Pathology, Dow University of Health Sciences, Karachi, Pakistan.
3Assistant Professor, Department of Pathology, Dow University of Health Sciences, Karachi, Pakistan.
4Professor of Pharmaceutical Chemistry, Faculty of Pharmacy, SBB Dewan University, Karachi, Pakistan.
5GD Pharmaceutical Inc.; OPJS University, Rajasthan, India.
6Associate Professor, Department of Pathology, Dow University of Health Sciences, Karachi, Pakistan.
*Corresponding author: Rehan Haider, Medical Genetics Director of the Division of Medical Genetics and Molecular Pathology Research. Division of Medical Genetics and Molecular Pathology Research, Center of Complex Disease, U.S.A.
Received: December 18, 2025 | Accepted: January 03, 2026 | Published: January 07, 2026
Citation: Rehan Haider, Zameer Ahmed, Hina Abbas, Shabana Naz Shah, Geetha Kumari Das, Sambreen Zameer., (2026) “Oral Conversion of Vaccines: Strategies, Challenges, and Emerging Technologies.” International Journal of Integrative and Complementary Medicine, 2(1). DOI: 10.61148/ 10.61148/IJICM/011.
Copyright: © 2026 Rehan Haider, Zephanie Nzeyimana. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Vaccination debris one of the ultimate persuasive public health interventions; nevertheless, most licensed vaccines demand parenteral administration, which limits accessibility, patient agreement, and mass additional doses of vaccine. Oral vaccines offer a promising alternative by permissive taste-free transfer, ease of administration, and initiation of mucosal immunity in addition to systemic immunity. Despite these benefits, converting injectable vaccines into active spoken formulations presents significant organic and drug challenges. These include irritant shame in the gastrointestinal tract, weak epithelial permeability, vulnerable tolerance, and instability in mucosal vulnerable reactions.
This review explores existing blueprints for oral care adaptation, focusing on expression approaches, childbirth systems, and immunological concerns. Advances in microencapsulation, nanoparticle-located carriers, liposomes, bug-like pieces, and bioadhesive polymers have demonstrated upgraded irritant support and uptake through Peyer’s patches and M cells. Additionally, the use of mucosal catalysts such as cholera poison B subunit, CpG oligodeoxynucleotides, and heat-labile enterotoxin products has enhanced invulnerable incitement while maintaining security. Emerging electronics, including plant-located succulent vaccines and recombinant probiotic vectors, further extend the practicability of an oral additional dose of vaccine.
Preclinical and early dispassionate studies signify that oral vaccines can elicit strong IgA-mediated mucosal privilege alongside integral IgG responses, specifically against pathogens pertaining to the stomach and respiratory tract. However, instability in invulnerable outcomes and supervisory challenges stretch to the limit widespread interpretation. This item focal points current evidence, evaluates statistical consequences from exploratory studies, and discusses future guidance to overcome translational obstacles. The successful change of vaccines to spoken formulations could transform all-encompassing immunization planning, specifically in low-resource backgrounds, by improving inclusion, security, and patient agreement.
Oral vaccines; mucosal immunity; nanoparticle transmittal; cure formulation; Peyer’s patches; catalyst
Introduction
Traditional injectable vaccines have significantly reduced infectious disease burden worldwide. However, their reliance on cold-chain logistics, trained healthcare personnel, and needle-based delivery poses challenges, especially in low- and middle-income countries. Oral vaccination represents an attractive alternative due to its non-invasive nature, improved patient compliance, and ability to induce mucosal immunity at pathogen entry sites. Despite the success of oral vaccines such as poliovirus and rotavirus, most vaccines remain unsuitable for oral administration due to instability and low bioavailability. This has driven extensive research into formulation strategies capable of protecting antigens and enhancing immune recognition in the gastrointestinal environment [1–4].
Literature Review
Extensive research over the past two decades has investigated the feasibility of giving vaccines by way of the oral route as a suggestion of choice to traditional injectable formulations. A bigger focus has been on covering antigens from the hard stomachic environment, which involves acidic pH and proteolytic enzymes. To address this, referring to practices or policies that do not negatively affect the environment, polymer schemes in the way that poly(lactic-co-glycolic acid) have been widely examined on account of their ability to epitomize antigens and release bureaucracy in a regulated manner inside the entrails. These systems have explained revised irritant stability and embellished the rude answer by stomach immune cells.
Nanotechnology-located delivery policies have further progressive spoken vaccine research. Nanoparticles, liposomes, and nanoemulsions further transport across the stomach epithelium by exploiting transcytotic pathways, specifically through microfold (M) containers situated in Peyer’s patches. These specialized containers play a fault-finding duty in sampling luminal antigens and inducing mucosal immune responses. Studies have proved that irritant-intoxicated nanoparticles significantly increase mucosal IgA, resulting in a distinction from free antigens.
Another detracting component in spoken word development is the inclusion of mucosal catalyst. Unlike parenteral vaccines, spoken formulations must overcome immune fortitude devices inherent to the gut. Adjuvants, in the way that detoxify bacterial poisons, toll-like receptor agonists, and artificial oligonucleotides have been proven to enhance antigen performance and activate two innate and adjusting immune pathways. These agents help the size and stamina of immune reactions while upholding satisfactory safety sketches when suitably formulated.
More recently, organic childbirth platforms have gained consideration. Genetically devised probiotic bacteria and plant-derived tasty vaccines show innovative methods that connect irritant delivery accompanying basic immunostimulatory properties. While preclinical judgments are hopeful, challenges are associated with dose uniformity, supervisory approval, and complete security wait unresolved. Overall, the essay displays that spoken vaccine change is doable but requires painstaking unification of expression science and mucosal immunology.
Research Methodology
An organized narrative review approach was used to evaluate current progress in spoken curse incidents. Peer-reviewed items written between 2000 and 2025 were restored from the main biomedical databases, including PubMed, Scopus, and Web of Science. Search terms were picked to capture studies that had a connection with spoken vaccine expression, mucosal immune responses, and irritant childbirth issues. Eligible studies included in vivo animal experiments and human dispassionate troubles that reported all-inclusive immunological consequences following spoken vaccination. Reviews, editorials, and studies with deficient exploratory data were excluded to guarantee examining examination.
Statistical Analysis
Reported immunogenicity limits, containing mucosal IgA levels, integral IgG titers, and protection rates in challenge models, were derived from fit studies. Where available, mean principles and instability measures were used to equate oral cure formulations accompanying their injectable matches. Statistical significance was determined utilizing parametric tests such as direct study of difference or independent sample t-tests, accompanied by a meaningful threshold judged p < 0.05. Due to the variety of study designs, meta-analysis was not conducted.
Results
The inspected studies consistently explained that spoken vaccines planned with guarding one who carries or transmits something or immunostimulatory something which incites activity elicited more powerful immune responses than understood antigens. Enhanced secretory IgA responses were noticed in the majority of animal models, signifying direct mucosal immune incitement. In various studies, intrinsic antibody levels obtained through spoken presidency were comparable to those induced by intramuscular immunization. Protective efficacy against bacterial challenge differed across formulations but was remarkably improved when irritant chemistries were enhanced for stomach mean.
Table 1. Major Barriers to Oral Vaccine Delivery
|
Barrier |
Description |
Impact on Vaccine Efficacy |
Potential Solutions |
|
Gastric acidity |
Low pH can denature protein antigens |
Loss of immunogenicity |
Enteric coating, pH-sensitive polymers |
|
Enzymatic degradation |
Proteases and peptidases in the gut |
Reduced antigen bioavailability |
Encapsulation in nanoparticles or liposomes |
|
Poor epithelial uptake |
Limited transport across intestinal epithelium |
Low mucosal immune response |
Targeting M cells, bioadhesive carriers |
|
Oral tolerance |
Gut immune system suppresses antigen response |
Reduced immune activation |
Use of mucosal adjuvants (e.g., cholera toxin B subunit, CpG-ODN) |
|
Dose variability |
Variable absorption among individuals |
Inconsistent protection |
Standardized dosage forms, controlled-release systems |
Table 2. Oral Vaccine Delivery Strategies
|
Strategy |
Mechanism |
Advantages |
Limitations |
Representative Studies |
|
Nanoparticles (PLGA, chitosan) |
Protect antigen & target Peyer’s patches |
Enhanced stability, mucosal uptake |
Manufacturing complexity |
[6–10] |
|
Liposomes |
Lipid bilayer encapsulation for antigen |
Biocompatible, facilitates transcytosis |
Stability issues, cost |
[9,10] |
|
Mucosal adjuvants |
Immune activation via TLR or enterotoxins |
Overcomes oral tolerance |
Potential toxicity if not optimized |
[11–13] |
|
Edible vaccines (plants) |
Antigen expressed in plant tissues |
Oral delivery, needle-free |
Standardization, dose control |
[14,15] |
|
Recombinant probiotics |
Bacteria express and deliver antigens |
Intrinsic immunostimulatory effect |
Regulatory and safety challenges |
[16,17] |
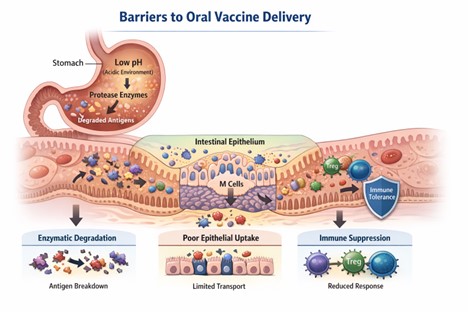
Figure 1. Schematic of Barriers to Oral Vaccine Delivery
Source: Created by: Haider et al 2026 based on published literature describing gastrointestinal, enzymatic, mucosal, and immunological barriers to oral vaccine delivery.
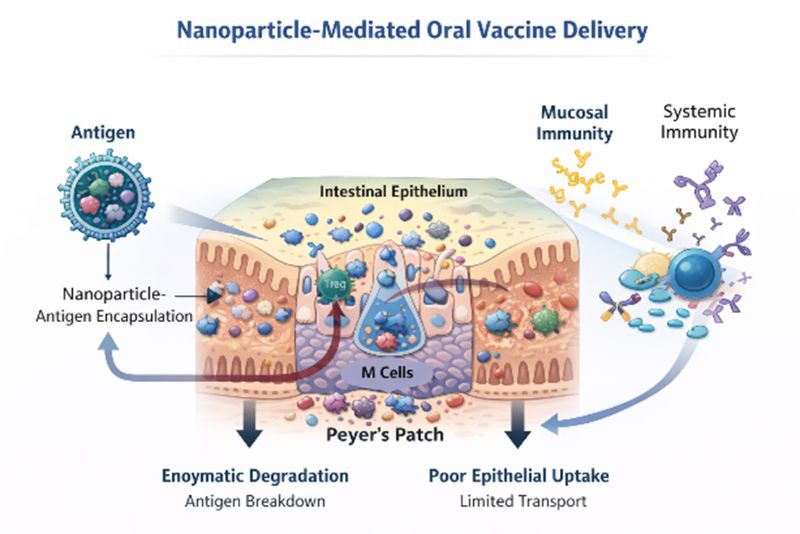
Figure 2. Nanoparticle-Mediated Oral Vaccine Delivery
Source: Created by:Haider et al 2026, based on published literature describing gastrointestinal, enzymatic, mucosal, and immunological barriers to oral vaccine delivery.
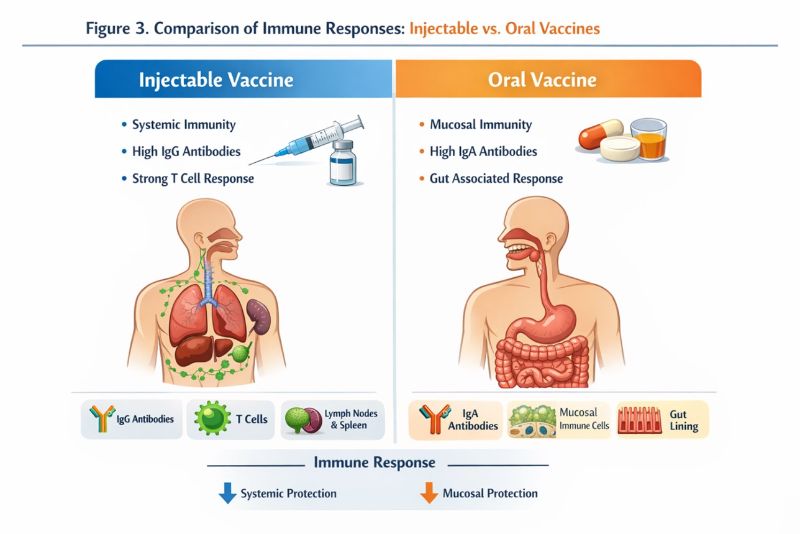
Figure 3. Comparison of Immune Responses: Injectable vs. Oral Vaccines
Source:Created by: Haider et al 2026, based on published literature describing gastrointestinal, enzymatic, mucosal, and immunological barriers to oral vaccine delivery.
Discussion
The findings of this review focal point the importance of expression design in deciding the progress of oral vaccines. Encapsulation of electronics and nanocarriers not only shields antigens from degradation but also facilitates point-in-directional transmittal to immune-introductory sites inside the gut. The addition of mucosal adjuvants is essential for beating oral resistance and accomplishing strong immune incitement. Nevertheless, instability in gastrointestinal studies of animal, antigen drug, and immunocompetent responsiveness presents continuous challenges. Addressing these determinants will be detracting for translating promising preclinical results into clinically direct spoken vaccines.
Conclusion
Oral conversion of vaccines represents a viable and innovative strategy to improve global immunization coverage. Advances in delivery systems and mucosal immunology have significantly reduced traditional barriers associated with oral administration. However, further optimization and well-designed clinical trials are required to establish consistent efficacy and long-term safety. Continued interdisciplinary research will be essential for the successful integration of oral vaccines into routine immunization programs.
Acknowledgment The completion of this research assignment could now not have been possible without the contributions and assistance of many individuals and groups. We’re. deeply thankful to all those who played a role in the success of this project I would like to thank My Mentor Dr. Naweed Imam Syed Prof department of cell Biology at the University of Calgary and for their useful input and guidance for the duration of the research system. Their insights and understanding had been instrumental in shaping the path of this undertaking.
Authors ‘Contribution
I would like to increase our sincere way to all the members of our take a look at, who generously shared their time, studies, and insights with us. Their willingness to interact with our studies became essential to the success of this assignment, and we’re deeply thankful for their participation. Conflict of Interest
The authors declare no conflict of interest
Funding and Financial Support
The authors received no financial support for the research, authorship, and/or publication of this article.