International Journal of Clinical Cardiology and Cardiovascular Interventions
OPEN ACCESS | Volume 4 - Issue 1 - 2025
ISSN No: 2836-2837 | Journal DOI: 10.61148/2836-2837/IJCCI
Patrizia Lo Sapio MD1*, Anna Maria Gori BS2, Niccolò Ciardetti MD2, Alessio Rossi MD2, Emiliano Chisci MD1, Nicola Troisi MD1, Elena Fanfani MD3, Stefano Michelagnoli MD1, Rossella Marcucci MD2
1Department of Surgery of Tuscany Center, San Giovanni di Dio Hospital, Florence, Italy
2Department of Experimental and Clinical Medicine, University of Florence, Italy
3Department of Anestesia and Intensive Care, San Giovanni di Dio Hospital, Florence, Italy
*Corresponding Author: Patrizia Lo Sapio, Department of Surgery of Tuscany Center, San Giovanni di Dio Hospital, Florence, Italy
Received date: November 07, 2021
Accepted date: November 10, 2021
Published date: November 16, 2021
Citation: Patrizia Lo Sapio, Anna Maria Gori BS, Niccolò Ciardetti, Alessio Rossi, Emiliano Chisci et al (2021) “Perioperative Management of Oral Anticoagulation: A Real-World Observational Study.” International J of Clinical Cardiology and Cardiovascular Interventions, 2(4); DOI: http;//doi.org/04.2021/1.1005.
Copyright: © 2021 Patrizia Lo Sapio. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Aims:Using a retrospective study, we evaluated: the risk of thromboembolism and bleeding following the perioperative management of oral anticoagulants (OACs) and the adherence to the guidelines by the clinicians involved.
Methods:Six hundred procedures, 120 for each OAC were collected from a Central Tuscany Surgery Department database.
The endpoints were:the 30-days rate of arterial, venous thrombotic events and bleedings, classified by ISTH, and their association with adherence to EHRA guidelines.
Results: Three hundreds and seventy one procedures(61%) were at high risk of bleeding.
Until 30 days of follow-up, thrombotic events occurred in 4 patients, 7% total bleedings;12.8% of bleedings occurred in inappropriate heparin bridging and 5.7% in patients without bridging (p <.016).
Four hundred and forty two (73.7%) procedures were performed in complete adherence to guidelines. At the multivariate analysis inappropriate bridging (HR=2.3;95% CI 1.1-4.7; p=.021) and urologic interventions (HR=2.3;95% CI 1.2-4.4;p=.01) were independent risk factors for bleeding events.
Conclusions: Bleedings were significantly correlated with inappropriate heparin bridging even if occurred also in correct management of OACs, being related to the major surgery itself.
Introduction
The increasing prevalence of atrial fibrillation (AF) in patients on oral anticoagulant (OAC) therapy that annually undergo invasive diagnostic or therapeutic procedures raised the problem of the perioperative management of direct oral anticoagulants (DOACs) (1, 2).
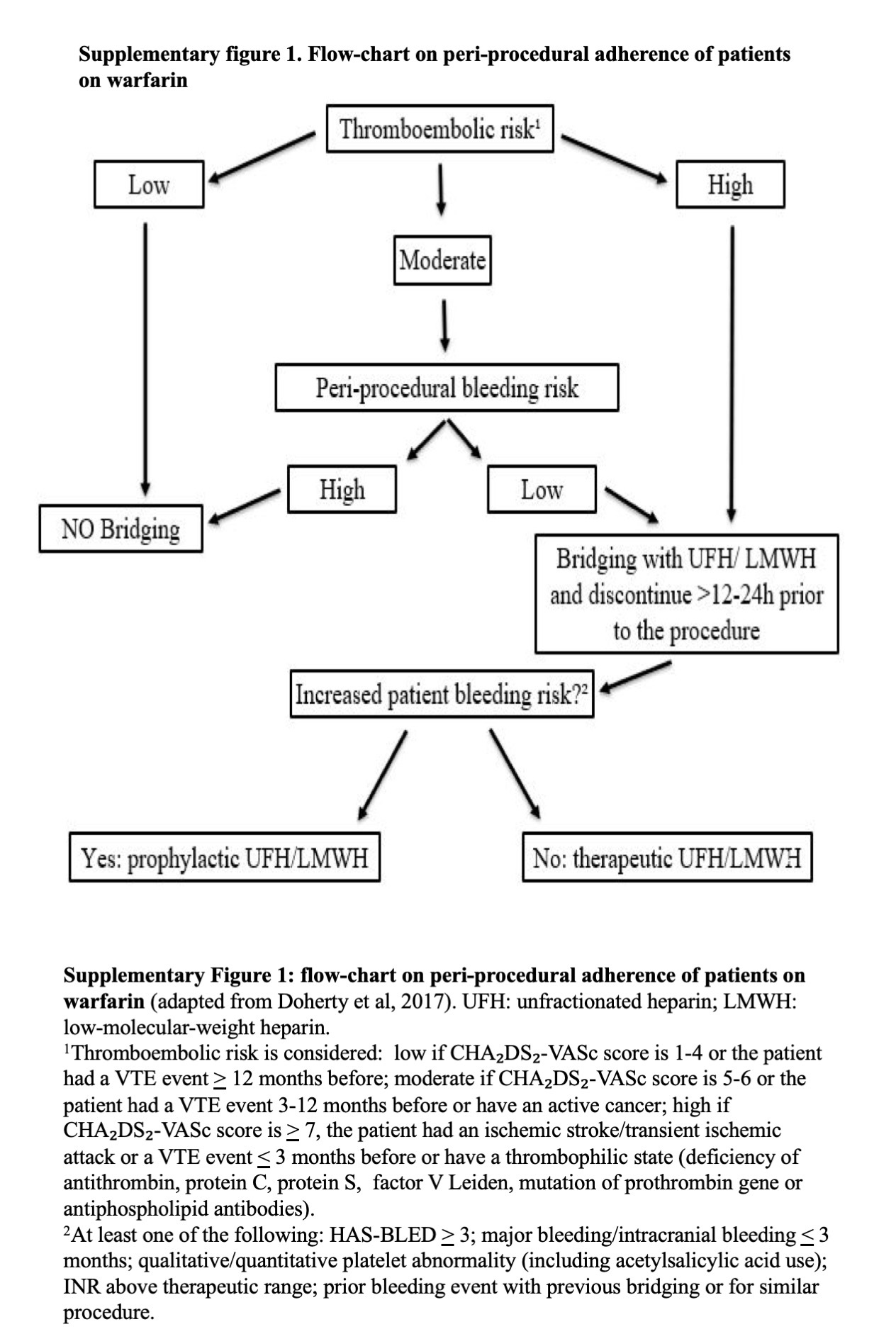
For the past 60 years we have used vitamin K antagonists (VKAs), which, due to their slow onset and offset characteristics, required the heparin bridging in pre-operative temporary interruption, even if recent evidences have shown the advisability of not bridging in patients with low-thromboembolic risk (3).
The progressive replacement of VKAs therapy with DOACs as first-line anticoagulant treatment has opened a new scenario but added complexity to the management of patients on OAC undergoing surgery (4-7). Indeed, the DOAC’s pharmacokinetic properties of short onset and offset action allow a short interruption interval and a post-procedural resumption without heparin bridging in patients undergoing invasive procedures (7-11).
The first clinical studies investigating perioperative DOACs regimen management derive from the retrospective sub-analyses of randomized clinical trials that assessed DOACs efficacy and safety for stroke prevention in AF (5-11). Only in the RELY study, a pre-procedural protocol of dabigatran interruption was introduced about half-way through the trial (12). The prospective Dresden Registry has been the first study to evaluate the effectiveness and safety of the peri-procedural management of dabigatran and rivaroxaban therapy in the daily care of an unselected cohort of patients. (13)
More recently the American and European Societies of Cardiology and Anesthesia have produced separate practical guides on the timing of the of VKA and DOAC- interruption and resumption before surgery and invasive procedures (14-17). Actually EHRA practical guide provide an unified and simplified approach in many clinical scenarios (18).
The most current evidence derives from the PAUSE trial where a default standardized protocol was applied to patients on DOACs undergoing elective surgery and from EMIT study that evaluated the safety of a discretional clinical management of edoxaban in a periprocedural setting (19-20).
The perioperative management of patients on OAC undergoing surgery is pertinent to a category of specialists, including the surgeon, the anesthesiologist, the cardiologist and it varies widely in clinical practice (14-19). Therefore, our aim was to evaluate the thrombotic and bleeding events following surgery and the correlation with the adherence to the guidelines by the clinicians involved.
Methods
Patient selection
This is a retrospective, observational study involving patients on OAC therapy with warfarin or DOACs who underwent an elective surgical or interventional procedure performed at the Department of Surgery of Central Tuscany (Italy) between July 7, 2014 and July 16, 2020. Patients were included if the following inclusion criteria were met: (1) OAC therapy for atrial fibrillation (AF) and/or for venous thromboembolism (VTE) - deep vein thrombosis (DVT), pulmonary embolism (PE) or both- (2) age > 18 years, (3) interruption of OAC before the procedure and resumption after that, (4) at least one pre-operative visit with a surgeon, an anesthesiologist or a cardiologist, (5) a collection of pre-operative blood tests, including hemoglobin, platelets and creatinine and (6) availability of follow-up of 30 days after the procedure. Patients with (1) moderate to severe mitral stenosis, those with (2) mechanical valve prostheses, those needing an (3) urgent procedure and those who underwent (4) cardiac surgery were excluded from the study. The study has been performed in accordance with the declaration of Helsinki.
Data collection
For all patients the following data were recorded: (1) age, (2) gender, (3) indication to OAC, (4) type and dosage of OAC, (5) comorbidities (previous transient ischemic attack/stroke, active cancer, chronic obstructive pulmonary disease [COPD], obstructive sleep apnoea [OSA], peripheral artery disease [PAD], ischemic heart disease, cardiomyopathies and valvular heart disease [except for moderate to severe mitral stenosis and mechanical prosthesis]), (6) cardiovascular risk factors (hypertension, diabetes mellitus, dyslipidaemia, smoking status, body mass index [BMI]) and (7) concomitant antiplatelet therapy. Glomerular filtration rate (GFR) was estimated with the Cockcroft-Gault formula.
In patients with AF the stroke risk was assessed with the CHA₂DS₂-VASc (congestive heart failure, hypertension, age > 75 years [2 points], diabetes mellitus, stroke [2 points], vascular disease, age 65-74 years and sex category) score and the bleeding risk was assessed with the HAS-BLED (uncontrolled hypertension, abnormal renal and/or hepatic function, stroke, bleeding history or predisposition, labile INR, elderly, drugs or excessive alcohol drinking) score, according to guideline recommendations (18).
Procedures and peri-procedural OAC management
Peri-procedural bleeding risk was estimated in accordance with the EHRA classification of elective surgical interventions (16): procedures were differentiated as having minor, low or high bleeding risk (Supplementary table 1)
For all patients we collected: (1) the number of days of interruption of OAC before the procedure, (2) any pre-procedural bridging with low-molecular-weight heparin (LMWH) and its dosage and (3) the time of resumption of OAC after the intervention.
Adherence to the EHRA guidelines on the preoperative management of DOACs (18) was evaluated and differentiated in three groups: (1) adherence to EHRA guidelines, (2) non adherence for longer or shorter OAC interruption than recommended and (3) no adherence for LMWH bridging.
For patients receiving warfarin, adherent management to ACCP Guidelines (14) was defined when: (1) the drug was interrupted 5 days before the procedure, (2) use of LMWH bridging from the day after interrupting warfarin to the day before the procedure, only in patients with high thromboembolic risk, irrespective of peri-procedural bleeding risk, or with moderate thromboembolic risk but low peri-procedural bleeding risk, (3) no LMWH bridging use in patients with low thromboembolic risk and in patients with moderate thromboembolic risk but undergoing an high bleeding risk procedure. A flow-chart on peri-procedural adherence of patients on warfarin is shown in the Supplementary Figure 1.
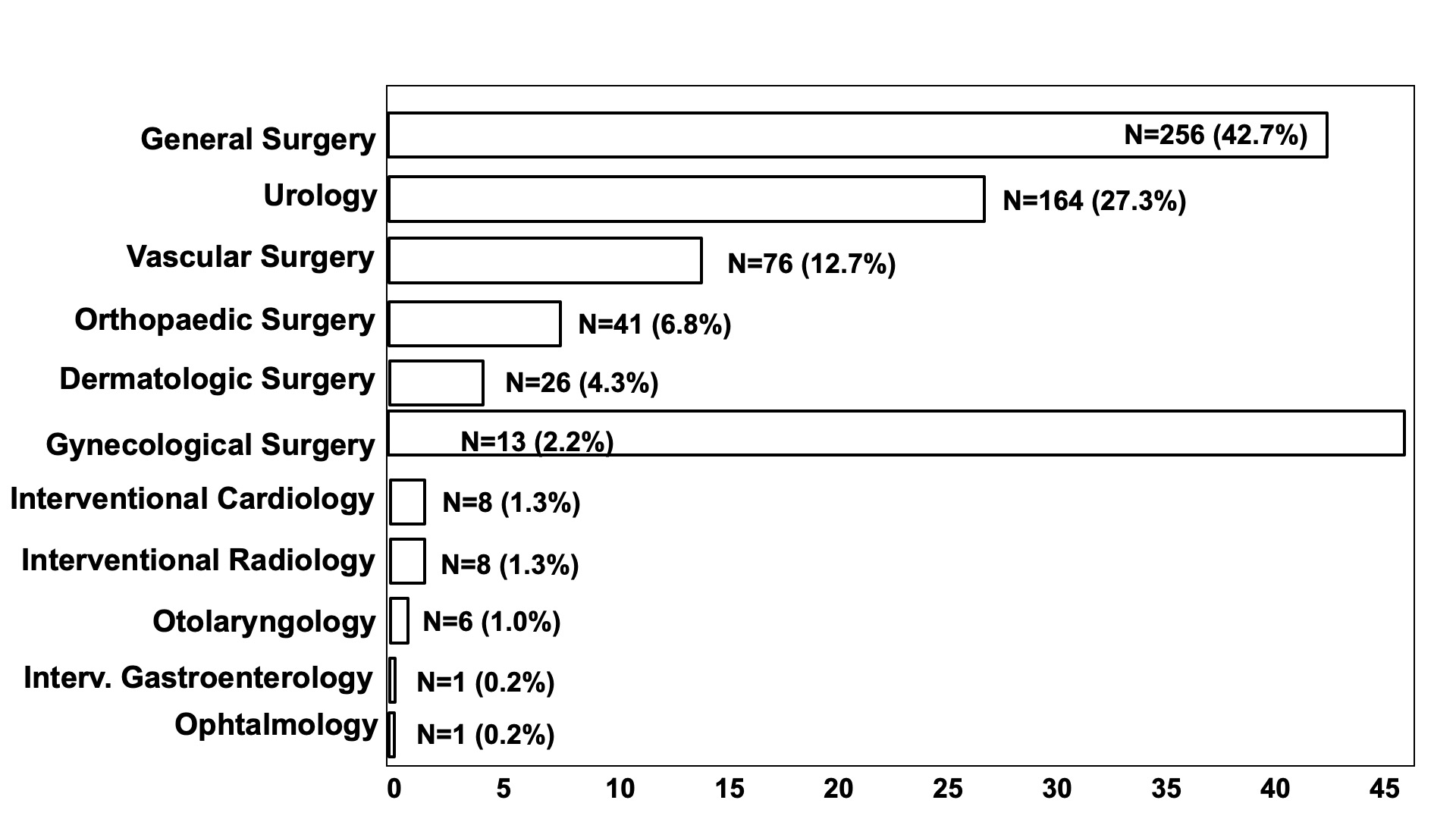
Figure 1: Types of procedures
Outcomes
The primary outcomes were:
Rates of outcomes were evaluated in the following 30 days after the procedure; clinical outcomes were stratified based on pre-procedural OAC management (1) adherence, 2) interruption time longer/shorter than recommended, 3) bridging with LMWH and based on type of OAC used.
Statistical Analysis
Continuous variables are presented as mean and standard deviation if they were normally distributed, as median and interquartile range if they had a non-Gaussian distribution. Categorical variables are presented as frequencies and percentages and were compared using χ2 test or Fisher’s exact test.
Event rates were based on Kaplan-Meier estimates in time-to-first-event analysis and a log-rank test was used for the evaluation of statistical significance.
Uni- and multi-variate analysis were performed using Cox regression analysis (backward model).
A P-value <0.05 was considered to indicate statistical significance.
All statistical analyses were carried out using the IBM® SPSS® Statistics (Statistical Package for Social Science, Chicago, Illinois, USA), Version 25.0.
Results
Patients and procedures
We evaluated a total of 600 procedures between July 7, 2014 and July 16, 2020. One hundred and twenty (20%) procedures were selected for each OAC taken. Baseline characteristics, comorbidities and cardiovascular risk factors of the population were displayed in Table 1. Types and dosages of different DOACs used are listed in Table 2. The indication for OAC therapy was stroke prevention in atrial fibrillation (n = 532; 88.7%), prior VTE event (n = 52; 8.7%) or both (n = 16; 2.7%). Prior VTE events were represented by PE (n = 6; 8.8%), DVT (n = 25; 36.8%) or both (n= 37; 54.4%).
The most common AF pattern was persistent/permanent (n = 346; 63.1%), compared to paroxysmal (n = 202; 36.9%). CHA₂DS₂-VASc score was > 2 in 535 (97.6%) procedures, with a mean value of 3,82 ± 1,42. HAS-BLED score mean value was 1.42 ± 0.74 (< 3 in 499 procedures; 91.1%) and most of the procedures had a high bleeding risk (n = 371; 61.8%), followed by low bleeding risk (n = 198; 33.0%) and minor bleeding risk (n = 31; 5.2%) interventions. Types of surgical and interventional procedures are shown in Figure 1.
OAC interruption was managed by anesthesiologists in 75.3% of procedures, by surgeons in 16% of procedures and by cardiologists in the remaining 8.7%.
Bleeding and thrombotic outcomes
Bleedings at 30 days of follow-up occurred in 42 (7%) patients. Of these, 29 (69%) were major bleedings and 13 (31%) were non-major bleedings, according to ISTH classification.
Details on major bleeding events are provided in Supplementary Table 3. Major bleedings occurred mainly after procedures with high bleeding risk, only in two procedures with low risk and in one with minor risk. Procedures associated with major bleeding events were: urologic procedures (n = 10; 34.5%), abdominal/pelvic surgery (n = 8; 27.6%), vascular interventions (n = 7; 24%), orthopaedic surgery (n = 2; 6.9%), proctological surgery (n = 1; 3.45%) and plastic surgery (n = 1; 3.45%). There was a case of retroperitoneal bleeding after a laparoscopic cholecystectomy, which required a surgical revision for haemostasis; other three bleeding events led to a re-operation: urgently needed on the same day after a carotid thromboendoarterectomy, on the first post-operative day after a parotidectomy and ten days after a hemorroidectomy surgery. One major bleeding from a femoral access for an endovascular aortic aneurysm repair brought to a surgical haemostasis at the patient’s bedside. Transfusion of ≥ 2 units of red cells was necessary in 9 (31%) major bleedings. Fortunately, no fatal and intracranial bleedings occurred.
Non-major bleeding events are listed in table Supplementary Table 4. Similar to major bleedings, also non-major bleedings happened mostly after urologic interventions (n = 9; 61.5%) and hematuria was the main type of bleeding event.
Rate of bleedings were more than doubled in patients who received inappropriate LMWH bridging compared to patients with complete adherence (12.8% vs 5.7%; p <.016). Rate of bleedings in patients with longer/shorter OAC interruption time wad 8.3%. Kaplan-Meier curves for bleedings stratified on pre-procedural OAC management are shown in Figures 2.
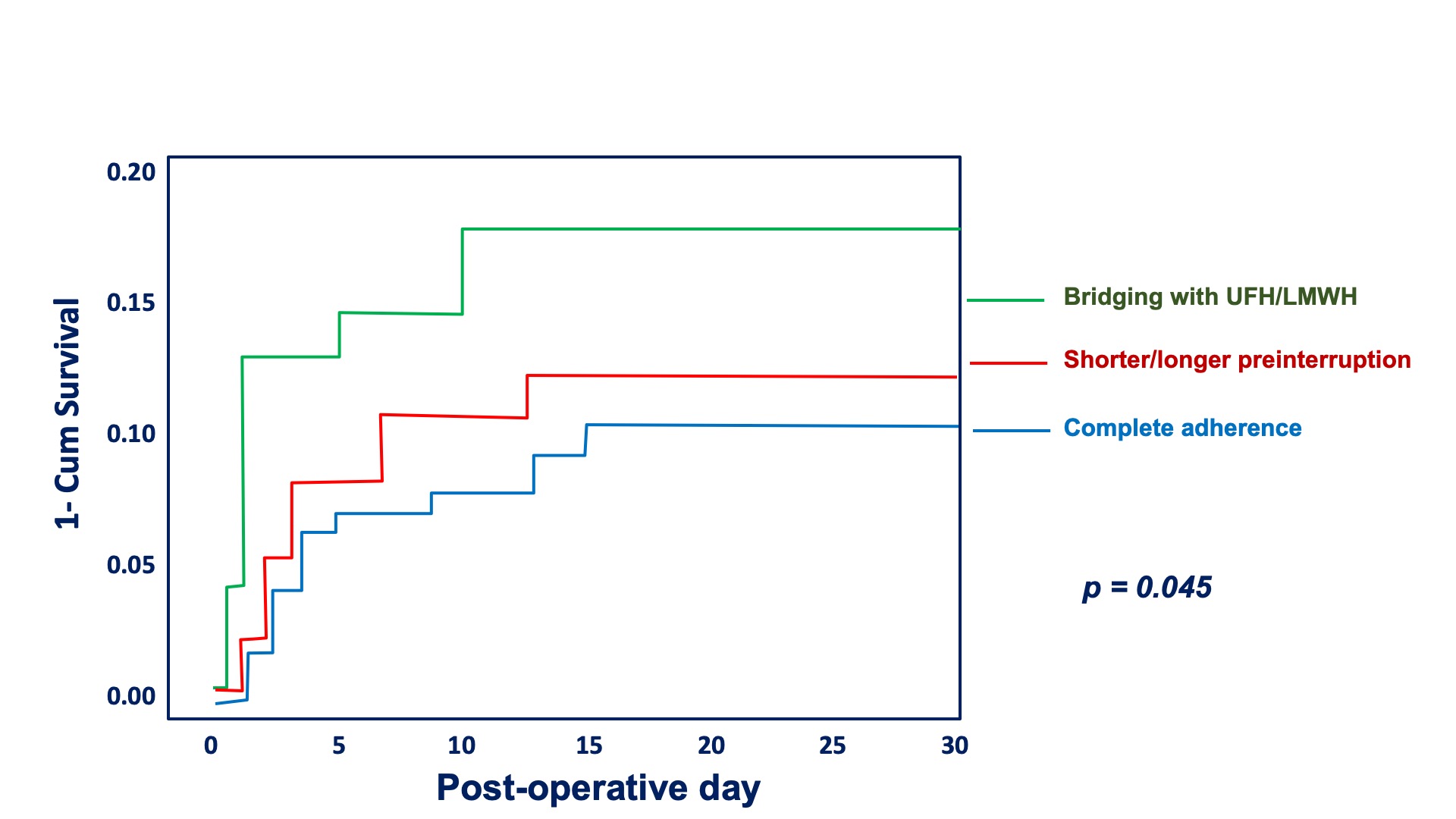
Figure 2: Kaplan-Meier curve for bleedings stratified on pre-procedural OAC management. Comparison between the three groups: patients completely adherent to guidelines/ recommendations (in blue), patients with shorter/longer interruption time (in red) and patients in whom bridging with LMWH was done (in green).
Thrombotic events occurred in 4/600 (0.7%) procedures, which consisted of two ischemic stroke, one acute peripheral arterial occlusion and one catheter-related internal jugular vein thrombosis. Detailed descriptions of the thrombotic events are provided in the Supplementary table 5.
Two of the four thrombotic events developed after a major bleeding. No statistically significant differences in thrombotic outcomes were observed between the three pre-procedural OAC management groups. There were no deaths in the period of follow-up.
Adherence to guidelines for pre-procedural OAC management
According to criteria above mentioned, adherence to guidelines/recommendations for pre-procedural OAC interruption was found in 442 (73.7%) procedures. OAC interruption time was longer than recommended in 25 (4.2%) procedures and shorter than recommended in 47 (7.8%) procedures. No adherence for LMWH bridging was documented in 86 (14.3%) procedures.
OAC type and outcomes
We did not detect any significant differences with regard to bleeding and thrombotic events between patients receiving warfarin or DOACs and among the four types of DOACs used (Supplementary Table 6).
Concomitant antiplatelet therapy
There were 63 (10.5%) patients who were taking a concomitant antiplatelet drug: 52 (82.5%) patients were taking acetylsalicylic acid (ASA) and 11 (17.5%) patients a P2Y12 inhibitor (ten patients were receiving clopidogrel and one ticlopidine); only one patient was on concomitant dual antiplatelet therapy. There were no statistically significant differences in terms of bleedings between patients who were taking a concomitant antiplatelet drug and those who were not ( Supplementary Table 7).
Predictors of bleeding
After uni- and multi-variate Cox regression analysis (Table 3), we found that independent risk factors for bleeding events were bridging with LMWH before the procedure (HR 2.3; 95% CI 1.1- 4.7; p = .021) and urologic interventions (HR 2.3; 95% CI 1.2- 4.4; p = .01). On the contrary, higher values of hemoglobin at baseline represented a protective factor for bleedings (HR 0.8; 95% CI 0.7- 1.0; p = .05).
Discussion
Data reported were obtained from a cohort of 600 procedures of patients on warfarin or DOACs for AF or VTE performed at the Department of Surgery of Central Tuscany (Italy).
The population studied had baseline characteristics comparable to those of other similar studies (12, 13, 19, 21,22,23), with a high cardiovascular and thrombotic risk profile and a high burden of comorbidities, as shown by 30.9% of active cancer.
In agreement with prior studies, we also found major bleeding event rates to be significantly higher after major procedures than after non-major procedures (12).
At variance with previous studies in the same setting - RELY 6.8% of major bleedings, Dresden Registry (5.3% of total bleedings), PAUSE (0.9 % of major bleedings for dabigatran, 1.3 % for apixaban, 1.8% for rivaroxaban), EMIT (total bleedings 4.2 % for edoxaban) - we found a higher rate of total bleedings (7% ) during the 30 days of follow-up. Among these , 69% were major bleedings, likely due to the higher hemorrhagic risk of the procedures evaluated (61.8%) with respect to the sub-analysis of RELY (17%), the Dresden Registry (10%) , the PAUSE cohort study (34.5%) and the EMIT prospective observational study (24%),(12,21,22, 23).
In the prospective dabigatran cohort study of the RELY, a standardized perioperative management approach was assessed in patients who required an elective surgery/procedure. Only 17% of patients had a major surgery and major bleeding were reported in 4.5% of patients (16% receiving heparin bridging). However, this study included only patients on dabigatran.
In the Dresden Registry, only 10% of patients, treated with rivaroxaban and dabigatran, underwent major surgery and major bleeding event rates were lower than in RELY (1.2 %), despite the fact that 30% of patients received heparin bridging.
The design of the PAUSE was different to the others because it assessed the safety of a standardized perioperative management based on DOAC-specific interruption and resumption intervals, without perioperative heparin bridging. Patients taking edoxaban and VKA were not included.
The EMIT study was a retrospective observational multicenter study on periprocedural management in patients on edoxaban therapy. The difference in the design of the EMIT study was that periprocedural management of edoxaban was at the discretion of the selected l investigators.
Our real-world analysis has confirmed that heparin bridging (12.8% vs 5.7%, respectively, p< 0.016) doubled bleeding events.
Indeed, at the multivariate analysis, heparin bridging (HR 2.3; 95%CI1.1-4.7; p<0.021) remained an independent risk factor for major bleeding, together with urologic interventions even though performed endoscopically (HR 2.3; 95% CI1.2-4.4; p<0.01).
Anyway, bleedings did occur in 5,7% of procedures, despite the management was adherent to the EHRA guidelines. This confirms the high bleeding risk of procedures included. attributable to the relevant tissue trauma inherent to the type of surgery and ,eventually, to the intraoperative heparin use ( i.e in major vascular surgery) .
The low number of thrombotic events did not allow a statistical analysis but half of them developed after an interruption of anticoagulant therapy due to a bleeding event.
No death for cardiovascular disease was observed in the population studied in the 30-day follow-up.
Our real-world observational study demonstrates a lack of adherence of clinicians to guidelines in 26.3 %, of procedures : for heparin bridging in 14.3 % , in 4.2% for longer interruption, in 7.8% for shorter interruption. These results are similar to those by Dresden Registry which reported a discrepancy with the expert recommendations of bridging in 30% of the procedures (19).
Conclusions
Although there are design differences , all studies show that a short-term interruption is the preferred approach in periprocedural anticoagulation management in order to minimize hemorrhagic or thromboembolic events.
In our retrospective observational study on periprocedural management of each OACs, including an unprecedented high risk population study either for the type of surgery or for the high hemorrhagic risk, the rate of total bleeding was high. The heparin bridging was a significant and independent risk factor, even if bleeding occurred also in correct management of OACs, being related to the major surgery itself. No bleeding was fatal.
These results results from a real-world data demonstrate that in the periprocedural OAC management:
1- heparin bridging is one of the most relevant reason for bleeding events;
- Coronary angiography;
- Electrophysiological study and catheter ablation;
- Pacemaker/ICD implantation;
- Inguinal/abdominal hernia surgery (without spinal/epidural anesthesia);
- Hemorrhoidal surgery;
- Breast-conserving surgery;
- Foot/hand surgery;
- Surgical procedure on varicose vein.
High bleeding risk procedures:
- Complex endoscopy;
- Abdominal and thoracic surgery;events;
2. In the clinical setting of major surgery, the bleeding risk is higher than the thrombotic one and inherent to the surgery (“per se”).
Supplementary Table1
Bleeding risk categories (adapted from Steffel J et al, 2018 and Shaw JR et al, 2020)
Minor bleeding risk procedures:
Low bleeding risk procedures:
High bleeding risk procedures:
Supplementary Table 2. ISHT classification of major bleedings (from Shulman S et al, 2005).
|
Major bleeding: |
|
Supplementary Table 3. Major bleedings.
A = apixaban; D= dabigatran; E = edoxaban; EVAR = endovascular aneurysm repair; OAC = oral anticoagulant; PTA = percutaneous transluminal angioplasty; R = rivaroxaban; URC= units of red cells; W= warfarin; TEA = thromboendarterectomy; TURBT = transurethral resection of bladder tumour; TURP = transurethral resection of prostate.
|
Age, gender |
Procedure |
EHRA Bleeding risk |
Site of bleeding |
Post-operative day |
Adherence to guidelines |
OAC (type and dose) |
ISTH major bleeding reason |
|
82, M |
Hemorrhoidal surgery |
High (spinal anesthesia) |
Rectum |
10 |
Inappropriate bridging |
E 60 |
Hb fall >2 g/dl; surgical revision |
|
79, M |
TURP |
High |
Haematuria |
5 |
Complete |
E 60 |
Hb fall >2 g/dl; >2URC trasfusion |
|
78, M |
Cholecystectomy- laparoscopic |
High |
Abdominal wall haematoma |
0 |
Complete |
R 20 |
Hb fall >2 g/dl |
|
75, M |
Hemicolectomy and cholecystectomy |
High |
Intestinal bleeding |
4 |
Complete |
A 2,5 |
Hb fall >2 g/dl; >2URC trasfusion |
|
85, F |
Urethral caruncle |
High |
Haematuria |
4 |
Complete |
A 2.5 |
Hb fall >2 g/dl; >2URC trasfusion |
|
83, F |
EVAR |
High |
Inguinal haematoma |
2 |
Complete – 5000 UI UFH during procedure |
D 110 |
Hb fall >2 g/dl; surgical haemostasis |
|
84, M |
Gastrectomy and cholecystectomy |
High |
Drainage |
2 |
IT shorter |
D 110 |
Hb fall >2 g/dl |
|
87, M |
Toe amputation |
Low |
Surgical wound |
1 |
Inappropriate bridging |
W |
Hb fall >2 g/dl |
|
80, F |
Femoral TEA + PTA iliac arteries |
High |
Inguinal haematoma |
1 |
Inappropriate bridging |
W |
Hb fall >2 g/dl; >2URC trasfusion |
|
72, M |
Iliac aneurismectomy |
High |
Inguinal haematoma |
1 |
Complete |
A 2.5 |
Hb fall >2 g/dl |
|
87, F |
Femoral TEA |
High |
Inguinal haematoma |
1 |
Complete |
E 30 |
Hb fall >2 g/dl; >2URC trasfusion |
|
82, F |
Anterior rectal resection |
High |
Rectal bleeding |
1 |
Complete |
E 60 |
Hb fall >2 g/dl |
|
85, M |
Knee arthroplasty |
High |
Surgical wound |
1 |
Inappropriate bridging |
D 110 |
Hb fall >2 g/dl |
|
78, F |
Knee arthroplasty |
high |
Surgical wound |
1 |
Complete |
D 150 |
Hb fall >2 g/dl; >2URC trasfusion |
|
83, M |
Parotidectomy |
Low |
Surgical wound haematoma |
1 |
Inappropriate bridging |
W |
Hb fall >2 g/dl; surgical revision |
|
77, M |
Laparoscopic haemicolectomy |
High |
Drainage |
1 |
Complete |
R 20 |
Hb fall >2 g/dl |
|
87, M |
TURBT |
High |
Haematuria |
1 |
Inappropriate bridging |
W |
Hb fall >2 g/dl |
|
73, M |
TURP |
High |
Haematuria |
1 |
Complete |
E 60 |
Hb fall >2 g/dl |
|
76, M |
TURBT |
High |
Haematuria |
19 |
Complete |
R 20 |
Hb fall >2 g/dl; premature resumption |
|
76, M |
Cistectomy |
High |
Haematuria |
12 |
IT longer |
R 20 |
Hb fall >2 g/dl |
|
77, M |
TURP |
High |
Haematuria |
11 |
Complete |
D 150 |
Hb fall >2 g/dl |
|
85, M |
Endoscopic urethrotomy |
Low |
Haematuria |
8 |
Complete |
W |
Hb fall >2 g/dl |
|
85, F |
TURBT |
High |
Haematuria |
3 |
IT longer |
W |
Hb fall >2 g/dl; >2URC trasfusion |
|
85, F |
TURBT |
High |
Haematuria |
1 |
IT longer |
W |
Hb fall >2 g/dl; >2URC trasfusion |
|
84, M |
Cistectomy |
High |
Haematuria |
1 |
Inappropriate bridging |
W |
Hb fall >2 g/dl; >2URC trasfusion |
|
74, F |
Cholecystectomy- laparoscopic |
High |
Retroperitoneal haematoma |
0 |
Inappropriate bridging |
R 20 |
Retroperitoneal bleeding; surgical revision |
|
73, M |
Carotid TEA |
High |
Cervical haematoma |
0 |
Inappropriate bridging |
D 110 |
Hb fall >2 g/dl; surgical revision |
|
89, M |
TURBT |
High |
Haematuria |
0 |
Inappropriate bridging |
A 5 |
Hb fall >2 g/dl |
|
74, M |
Dermatologic cancer surgical excision |
Minor |
Surgical wound |
0; 4 |
Complete |
E 30 |
Hb fall >2 g/dl; >2URC trasfusion |
Supplementary Table 4. Non-major bleedings.
A=apixaban; D=dabigatran; E=edoxaban; EVAR=endovascular aneurysm repair; OAC=oral anticoagulant; PTA=percutaneous transluminal angioplasty; R=rivaroxaban; URC=units of red cells; W=warfarin; TEA=thromboendarterectomy; TURBT=transurethral resection of bladder tumour; TURP=transurethral resection of prostate.
|
Age, gender |
Procedure |
bleeding risk |
Site of bleeding |
Post-operative day |
Adherence to guidelines |
OAC (type and dose) |
|
84, M |
TURBT |
High |
Haematuria |
14 |
Complete |
D 110 |
|
74, M |
Inguinal hernia |
Low |
Inguinal haematoma |
10 |
Complete |
A 5 |
|
86, M |
TURBT |
High |
Haematuria |
10 |
Complete |
E 30 |
|
81, M |
TURBT |
High |
Haematuria |
6 |
IT shorter |
D 110 |
|
81, M |
TURBT |
High |
Haematuria |
3 |
Complete |
A 2.5 |
|
73, M |
TURP |
High |
Haematuria |
3 |
Complete |
D 110 |
|
80, M |
PM implantation |
Low |
Pocket haematoma |
2 |
Complete |
R 15 |
|
72, M |
TURBT |
High |
Haematuria |
2 |
Complete |
A 5 |
|
87, M |
Inguinal hernia |
Low |
Inguinal haematoma |
1 |
IT shorter |
D 110 |
|
73, M |
Popliteal aneursimectomy |
High |
Haematuria |
1 |
Complete |
A 2.5 |
|
91, M |
TURBT |
High |
Haematuria |
5 |
inappropriate bridging before |
W |
|
81, M |
TURBT |
High |
Haematuria |
6 |
Complete |
A 2.5 |
|
86, M |
Cholecystectomy- laparoscopic |
High |
Surgical wound |
0 |
Complete |
R 15 |
Table 5. Descriptions of the thrombotic events
The patient was a 73 years old male. He was taking dabigatran 110 mg b.i.d. for stroke prevention in permanent atrial fibrillation. His cardiovascular risk factors were: overweight (BMI 26.4 kg/m2), hypertension, diabetes mellitus, dyslipidaemia, chronic kidney disease (GFR 44 ml/min) and a former smoking status. His CHA₂DS₂-VASc score was 6 and HAS-BLED score was 4. He underwent to a carotid thromboendarterectomy (high bleeding risk intervention). Blood tests prior to the procedure showed mild anaemia (Hb 12.9 g/dl) and normal platelet count (196 x 103/μL). OAC interruption time was shorter than recommended (dabigatran was interrupted 3 days before the procedure; EHRA recommend to stop dabigatran at least 96 hours before in case of GFR < 49 ml/min) and bridging with LMWH was done. A major bleeding occurred immediately after the procedure (cervical hematoma) and ischemic stroke occurred on fourth post-operative day.
The patient was a 74 years old male. He was taking apixaban 5 mg b.i.d. for stroke prevention in paroxysmal atrial fibrillation. His cardiovascular risk factors were: hypertension, dyslipidaemia and a former smoking status. He had a history of ischemic heart disease and COPD. His CHA₂DS₂-VASc score was 7 and HAS-BLED score was 3. He was planned to undergo to a carotid thromboendarterectomy (high bleeding risk procedure). Blood tests prior to the procedure showed mild anaemia (Hb 13.2 g/dl), normal platelet count (170 x 103/μL) and a GFR of 72 ml/min. Apixaban was interrupted approximately 3 weeks before the procedure (interruption time longer than recommended) and dual antiplatelet therapy with ASA and clopidogrel was initiated. After that, a cardioembolic stroke occurred and the patient underwent to a carotid artery stenting the day after, instead of the planned carotid TEA.
The patient was a 68 years old male. He was on warfarin for stroke prevention in permanent atrial fibrillation. His cardiovascular risk factors were: hypertension, dyslipidaemia and he had a history of PAD. His CHA₂DS₂-VASc score was 5 and HAS-BLED score was 4. He underwent to a PTA of lower limb peripheral arteries (low bleeding risk intervention). Blood tests prior to the procedure showed anaemia (Hb 11.2 g/dl) and thrombocytopenia (148 x 103/μL) and a GFR of 68 ml/min. Pre-procedural OAC management was done according to guidelines (complete adherence) and so was resumption of OAC (warfarin was resumed immediately after the intervention, with LMWH bridging). An acute peripheral arterial occlusion nevertheless occurred on the thirteenth post-operative day.
The patient was a 77 years old male. He was on rivaroxaban 20 mg q.d. for a previous VTE event that occurred two years before. His cardiovascular risk factors were: overweight (BMI 29.4 kg/m2), hypertension, dyslipidaemia and a former smoking status. He had an active colon cancer for which he underwent to a hemicolectomy (high bleeding risk intervention). Blood tests prior to the procedure showed anaemia (Hb 11.1 g/dl), normal platelet count (303 x 103/μL) and a GFR of 60 ml/min. Pre-procedural management of OAC was done according to guidelines (complete adherence): rivaroxaban was interrupted 3 days before the procedure and no bridging with UFH/LMWH was done. A major bleeding nevertheless occurred in first post-operative day. For this reason, rivaroxaban was resumed only two weeks after the intervention and on the ninth post-operative day a diagnosis of catheter related internal jugular vein thrombosis was done.
Supplementary Table 6. Thrombotic and bleeding events according to type of OA
Supplementary Table 7. Thrombotic and bleeding events (combined) stratified on concomitant antiplatelet therapy
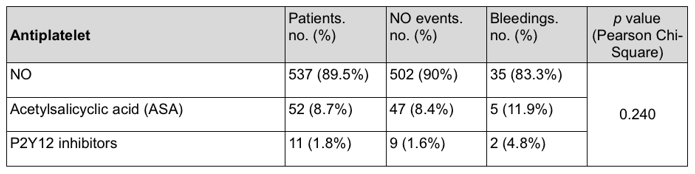
Table 1: Baseline characteristics, comorbidities and cardiovascular risk factors of the population.
|
Mean age (yr), mean ± SD |
76.6 ± 8.7 |
|
Female sex, n (%) |
202 (33.7%) |
|
BMI (Kg/m²), mean ± SD |
26.3 ± 4.1 |
|
Haemoglobin (g/dl), mean ± SD |
13 ± 1.7 |
|
Platelets (10³/µl), mean ± SD |
219 ± 69.8 |
|
Creatinine (mg/dl), mean ± SD |
0.98 ± 0.33 |
|
GFR (ml/min), mean ± SD |
69.6 ± 25.7 |
|
TIA or stroke, n (%) |
40 (6.7%) |
|
Active cancer, n (%) |
185 (30.9%) |
|
COPD, n (%) |
125 (20.8%) |
|
OSA, n (%) |
15 (2.5%) |
|
PAD, n (%) |
115 (19.2%) |
|
Ischemic heart disease, n (%) |
135 (22.5%) |
|
Cardiomyopathies, n (%) |
38 (6.3%) |
|
Valvular heart disease*, n (%) |
61 (10.2%) |
|
Hypertension, n (%) |
483 (80.5%) |
|
Uncontrolled hypertension (systolic >160 mmHg), n (%) |
33 (5.5%) |
|
Diabetes mellitus, n (%) |
118 (19.7%) |
|
Dyslipidaemia, n (%) |
226 (37.7%) |
|
Current/ former smokers, n (%) |
63 (10.5%) / 212 (35.7%) |
*Except for moderate to severe mitral stenosis and mechanical prosthesis.
BMI= body mass index; COPD= chronic obstructive pulmonary disease; GFR= glomerular filtration rate; OSA= obstructive sleep apnoea; PAD= peripheral artery disease; TIA= transient ischemic attack.
Table 2: Types and dosages of different DOACs.
|
DOAC: type and dosage |
n (%) |
|
Apixaban 2.5 mg bid |
48 (8%) |
|
Apixaban 5 mg bid |
72 (11.9%) |
|
Dabigatran 110 mg bid |
59 (9.8%) |
|
Dabigatran 150 mg bid |
61 (10.2%) |
|
Edoxaban 30 mg qd |
35 (5.8%) |
|
Edoxaban 60 mg qd |
85 (14.1%) |
|
Rivaroxaban 15 mg qd |
46 (7.7%) |
|
Rivaroxaban 20 mg qd |
74 (12.3%) |
bid = bis in die; qd = quaque die.
Table 3: Bleeding events in patients on warfarin vs DOACs.
|
Anticoagulant |
Without Bleeding Events, n (%) |
Bleedings Events, n (%) |
p value |
|
Warfarin |
111 (19.9%) |
9 (21.4%) |
|
|
Apixaban |
111 (19.9%) |
9 (21.4%) |
|
|
Edoxaban |
110 (19.7%) |
10 (23.8%) |
0.921 |
|
Dabigatran |
113 (20.3%) |
7 (16.7%) |
|
|
Rivaroxaban |
113 (20.3%) |
7 (16.7%) |
|
Table 4: Thrombotic events in patients on warfarin vs DOACs.
|
Anticoagulant |
Without Thrombotic events, n (%) |
Thrombotic Events, n (%) |
p value |
|
Warfarin |
119 (20%) |
1 (25%) |
|
|
Apixaban |
119 (20%) |
1 (25%) |
|
|
Edoxaban |
119 (20%) |
1 (25%) |
0.909 |
|
Dabigatran |
120 (20.1%) |
0 (0%) |
|
|
Rivaroxaban |
119 (20%) |
1 (25%) |
|
Table 5: Bleeding events stratified on concomitant antiplatelet therapy.
|
Antiplatelet |
Patients, n (%) |
Without Bleeding Events, n (%) |
Bleedings Events, n (%) |
p value |
|
NO |
537 (89.5%) |
502 (90%) |
35 (83.3%) |
|
|
Acetylsalicyclic acid (ASA) |
52 (8.7%) |
47 (8.4%) |
5 (11.9%) |
0.240 |
|
P2Y12 inhibitors |
11 (1.8%) |
9 (1.6%) |
2 (4.8%) |
|
Table 6: Univariate and multivariate Cox regression analysis of bleeding events.
|
Characteristic |
Hazard ratio (95% C.I.) |
p value |
Hazard ratio (95% C.I.) |
p value |
|
Bridging with LMWH |
2.30 (1.14-4.66) |
0.021 |
2.22 (1.12-4.43) |
0.023 |
|
Years of age |
1.03 (0.98-1.09) |
0.232 |
1.07 (1.02- 1.11) |
0.003 |
|
Female sex |
0.40 (0.18-0.90) |
0.027 |
0.53 (0.25-1.11) |
0.093 |
|
Glomerular filtration rate (GFR) |
0.99 (0.97-1.01) |
0.285 |
0.98 (0.97-0.99) |
0.006 |
|
Hemoglobin (g/dl) |
0.82 (0.68-1.00) |
0.050 |
0.79 (0.66-0.94) |
0.009 |
|
Urologic interventions (yes/no) |
2.31 (1.23-4.36) |
0.010 |
2.44 (1.33-4.48) |
0.004 |
|
CHA₂DS₂-VASc (per point) |
1.02 (0.79-1.31) |
0.908 |
1.14 (0.92-1.40) |
0.230 |
|
HAS-BLED > 3 |
1.51 (0.59-3.85) |
0.394 |
1.98 (0.83-4.69) |
0.122 |
Conflict of interests
All Authors disclose any financial associations that might pose a conflict of interest in connection with the submitted article.