International Journal of Clinical Cardiology and Cardiovascular Interventions
OPEN ACCESS | Volume 4 - Issue 1 - 2025
ISSN No: 2836-2837 | Journal DOI: 10.61148/2836-2837/IJCCI
Adrian Carlessi 1*, Leonel Perello 1, Cristian Pantaley2, Armando Borsini 2
1Department of Cardiovascular Imaging. Hospital Jose Maria Cullen 2150 Freyre Street Santa Fe, Santa Fe, Argentina.
2Department of Cardiology. Hospital Jose Maria Cullen 2150 Freyre Street Santa Fe, Santa Fe, Argentina.
3Department of Pneumatology and Allergy Hospital Jose Maria Cullen 2150 Freyre Street, PC 3000 Santa Fe, Santa Fe, Argentina.
4Medicine School, Litoral National University Route 168, 80, Santa Fe, Santa Fe, Argentina.
5Department of Medical Images. Hospital Jose Maria Cullen 2150 Freyre Street Santa Fe, Santa Fe, Argentina.
*Corresponding Author: Adrian Carlessi, Department of Cardiovascular Imaging Hospital Jose Maria Cullen, 2150 Freyre Street, Santa Fe, Santa Fe, Argentina.
Received date: October 22, 2021
Accepted date: October 25, 2021
published date: October 29, 2021
Citation: Adrian Carlessi, Leonel Perello, Cristian Pantaley, Armando Borsini , Lucia Rossi et al. (2021) “Analysis of Cardiac Involvement in Patients Recovered from Covid-19 Without Troponin Elevation, Evaluated by Cardiovascular Magnetic Resonance.” International J of Clinical Cardiology and Cardiovascular Interventions, 2(4); DOI: http;//doi.org/04.2021/1.1003.
Copyright: © 2021 Adrian Carlessi. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background: The disease caused by coronavirus disease 2019 (COVID-19) affects the cardiovascular system, whether by direct viral aggression or indirectly through systemic inflammation and multiple organ compromise. A widely used method to determine cardiac injury is troponin measurement. The aim of this study was to evaluate the prevalence of cardiac involvement (CINV) in a population recovered from COVID-19 referred to cardiac MRI (CMR) who did not present troponin elevation.
Methods: There were 156 patients who recovered from COVID-19 and who did not present troponin elevation referred to CMR. CINV was considered to be the presence of: late gadolinium enhancement (LGE), edema, myocarditis, pericarditis, left ventricular systolic dysfunction (LVSD) and/or depressed right ventricular systolic dysfunction (RVSD).
Results: The prevalence of CINV was 28.8%, being more frequent in men (p=0.002), in patients who required hospitalization (p=0.04) and in those who experienced non-mild cases of infection (p=0.007). RVSD (17.9%) and LVSD (13.4%) were the most frequent findings. The rate of myocarditis was 0.6%. LGE manifested in 7.1% of patients and its presence was related to less left ventricular ejection fraction (LVEF) (p=0.0001) and right ventricular ejection fraction (RVEF) (p=0.04).
Conclusion: In patients who recovered from COVID-19, 28.8% CINV was found. It was more frequent in men, in patients who required admission and in patients with cases of non-mild infection. The patients who presented LGE had less LVEF and RVSF.
Introduction
Coronavirus disease 2019 (COVID-19), caused by the coronavirus of severe acute respiratory syndrome (SARS-CoV-2), emerged in Wuhan, China, in December 2019 in patients who presented with pneumonia of unknown origin[1]. Since then and to date, when this paper is written, 134,719,328 cases of COVID-19 have developed worldwide, with at least 2,915,972 deaths[2]. Although COVID-19 mainly presents with respiratory system compromise, due to the interaction between COVID-19 and the cardiovascular system[3-4], cardiac injury is frequently observed in these patients. The mechanism of cardiac injury is multifactorial and includes oxygen supply-demand imbalance without coronary obstruction, myocardial stress, inflammation, microvascular dysfunction, pre-existing atherosclerotic plaque rupture or toxicity by direct viral injury[5]. Studies show a high prevalence of cardiovascular alterations in hospitalized patients[6-7]. Cardiac injury determination through high-sensitivity cardiac troponin shows a worse evolution in patients with an increase in this biomarker[8-9].
However, the impact on the heart in the mid-term published in different studies is controversial. In the initial reports, a high prevalence of cardiac disease was shown in cardiovascular magnetic resonance (CMR), with elevation in native T1 and T2 mapping being the most frequent finding. These studies included both patients with and without troponin elevation. Late gadolinium enhancement (LGE) was approximately 31-32% [10-11]. On the other hand, it was recently observed in patients who were admitted and had elevated troponins at hospital discharge, that the LGE prevalence was 33% of nonischemic LGE, which may be attributed to COVID-19[12].
The aim of our study was to determine the prevalence of LGE abnormalities in a large sample of patients recovered from COVID-19, who did not present troponin elevation at the time of the study.
Methods
This is an observational, prospective, single-center study, performed at the Hospital JM Cullen of the city of Santa Fe, Argentina. Consecutive patients from October 2020 to April 2021 who were initially referred for an LGE test and who met the following inclusion criteria were included: 1) signed informed consent 2) confirmed to have infection by SARS-CoV-2 by reverse transcription polymerase chain reaction (RT-PCR); 3) more than four weeks after epidemiological discharge; and 4) not presenting troponin elevation at the time of the study. The exclusion criteria were as follows: 1) refusal to sign the informed consent form; 2) chronic kidney disease with creatinine clearance ≤30 ml/min; 3) device or prosthesis not compatible with CMR; and 4) claustrophobia. The protocol was analyzed and approved by the institutional bioethical review board. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's human research committee.
According to the severity of COVID-19 symptoms reported by the patient and considering the maximum level of healthcare required, acute infection was categorized into: mild, moderate, severe and critical, according to the Ordinal Scale for Clinical Improvement (OSCI) proposed by the World Health Organization[13].
CRM protocol and post-process
The analysis was performed by Cardiac YX (GE Healthcare) for the evaluation of LV volumes and mass, while trabeculae and papillary muscles were excluded. The presence of LGE, edema, myocarditis, pericarditis, left ventricular systolic dysfunction (LVSD) and/or right ventricular systolic dysfunction (RVSD) was classified as cardiac involvement (CINV). Active myocarditis was defined as the presence of nonischemic LGE (subepicardial or mesocardial location) associated with the edema criterion in T2-STIR sequences and/or hyperemia in early postcontrast T1 sequences. The criterion of myocardial edema was established through the myocardial edema index (EI), which was defined as the relationship between myocardial signal intensity (SI) and skeletal muscle SI[14]. An EI >2.0 was considered abnormal. The pericarditis criterion was the presence of pericardial LGE or pericardial thickening (≥4 mm) associated with pericardial effusion. LVSD was determined by left ventricular ejection fraction (LVEF) when it was less than 57% and RVSD when right ventricular ejection fraction (RVEF) was less than 51% in women and 52% in men[14]. The location and pattern of LGE injuries in LGE images were evaluated by 2 observers, who reviewed all PSIR images independently.
Statistical analysis
Statistical analyses were made with IBM SPSS v23. Normality of quantitative variables was evaluated by the Shapiro-Wilk test. To describe quantitative variables, means or medians were used as central tendency measures and standard deviations (SDs) or interquartile ranges (IQRs) were used as corresponding dispersion measures. Qualitative variables are presented as absolute frequencies and their respective percentages. Independent samples t-tests were used to evaluate differences in means between two groups. In the case of not meeting normality criteria, the Mann-Whitney U test was used to compare distributions. Fisher’s exact tests and Pearson’s chi-squared tests were used to compare differences in rates between qualitative variables. Correlations between quantitative variables were evaluated by means of the Pearson correlation coefficient. Confidence intervals of 95% (95% CI) were attached to the parameters when deemed necessary. α statistical significance was established as 0.05.
Results
Clinical characteristics.
The baseline characteristics of the patients are shown in Table 1. There were 156 patients included, of whom 55.8% were men. Age average was 48.4 (SD 13.3). There were 58.9% of patients with a mild evolution in the acute phase of the disease; only 5.9% of patients were severe/critical. Patients that required admission were 35.2%. The median and IQR of hospital stay was 10 (5-15) days; while for the ICU, it was 6 (4-20) days. Twenty-one percent of patients reported presenting atypical precordial pain, 18% palpitations and 515 shortness of breath when undergoing CMR. All these symptoms were not incapacitating and no case required admission.
Table1. Clinical characteristics and CMR findings. COPD: Chronic Obstructive Pulmonary Disease; DBT: Diabetes; RVEF: Right Ventricular Ejection Fraction; LVEF: Left Ventricular Ejection Fraction; LVMI: Left Ventricular Mass Index; RVEDVI: Right Ventricular End Diastolic Volume Index; LVEDVI: Left Ventricular End Diastolic Volume Index; RVESVI: Right Ventricular End Systolic Volume Index; LVESVI: Left Ventricular End Systolic Volume Index; RVSVI: Right Ventricular Systolic Volume Index; LVSVI: Left Ventricular Systolic Volume Index.
|
Variable |
|
|
- Clinical characteristics |
Value |
|
Age mean ± SD |
48.4 ± 13.3 |
|
Male gender n (%) |
87 (55.8) |
|
Body mass index mean ± SD |
28.4 ± 5.3 |
|
HTN n (%) |
54 (34.6) |
|
DBT n (%) |
22 (14.1) |
|
Smoker n (%) |
48(30.8) |
|
COPD n (%) |
5 (3.2) |
|
Previous CAD n (%) |
5 (3.2) |
|
Previous stroke n (%) |
2 (1.3) |
|
Charlson comorbidity score media ± SD |
0.88 ± 1.37 |
|
Admission due to COVID-19 n (%) |
55 (35.3) |
|
COVID-19 severity |
|
|
Mild n (%) |
92 (59) |
|
Moderate n (%) |
55 (35.2) |
|
Severe/critical n (%) |
9 (5.8) |
|
Systolic blood pressure in mmHg mean ± SD |
123.8 ± 16.2 |
|
Diastolic blood pressure in mmHg mean ± SD |
74.9 ± 9.3 |
|
Heart rate in bpm mean ± SD |
73.1 ± 14.4 |
|
- CMR findings |
|
|
Days since epidemiological discharge to CMR median (IQR) |
58.5 (44-82) |
|
LVEDVI in ml/m2 mean ± SD |
69.1 ± 15.6) |
|
LVESVI in ml/m2 mean ± SD |
26.2 ± 9.3 |
|
LVSVI in ml/m2 mean ± SD |
42.8 ± 8.6 |
|
LVEF (%) mean ± SD |
62.2 ± 6.5 |
|
LVMI in g/m2 mean ± SD |
48.3 ± 10.6 |
|
RVEDVI in ml/m2 mean ± SD |
71.5 ± 16.4 |
|
RVESVI in ml/m2 mean ± SD |
30.5 ± 9.4 |
|
RVSVI in ml/m2 mean ± SD |
40.1 ± 9.8 |
|
RVEF in ml/m2 mean ± SD |
57.5 ± 6.7 |
CMR finding.
The mean and IQR of time from the end of symptoms until performing CMR was 58.5 (44-82) days. In 28.8% of patients, CINV was observed. Figure 1 shows the prevalence of the different alterations constituting CINV. LVSD and RVSD, with 13.5% and 17.9% respectively, were the most frequent alterations. Myocardial LGE findings were observed in 11 patients (7.1%) and were the most frequent variable found to be myocardial tissue involvement. Table 2 shows the characteristics of patients according to the CMR findings. A statistically significant difference was found for gender, with CINV being more prevalent in men (39.1% vs 15.9%, p=0.002). Patients hospitalized due to COVID-19 presented a higher CINV prevalence than those who stayed at home through the disease (38.2% vs 23.7%, p=0.04). In turn, patients who developed non-mild COVID-19 had more CINV than those with mild cases (40.7% vs 20.6%, p=0.007). No difference was observed in the median of days from epidemiological discharge to performance of CMR between the groups, 54 (44-82) days vs 62 (43-84) days (p=0.41).
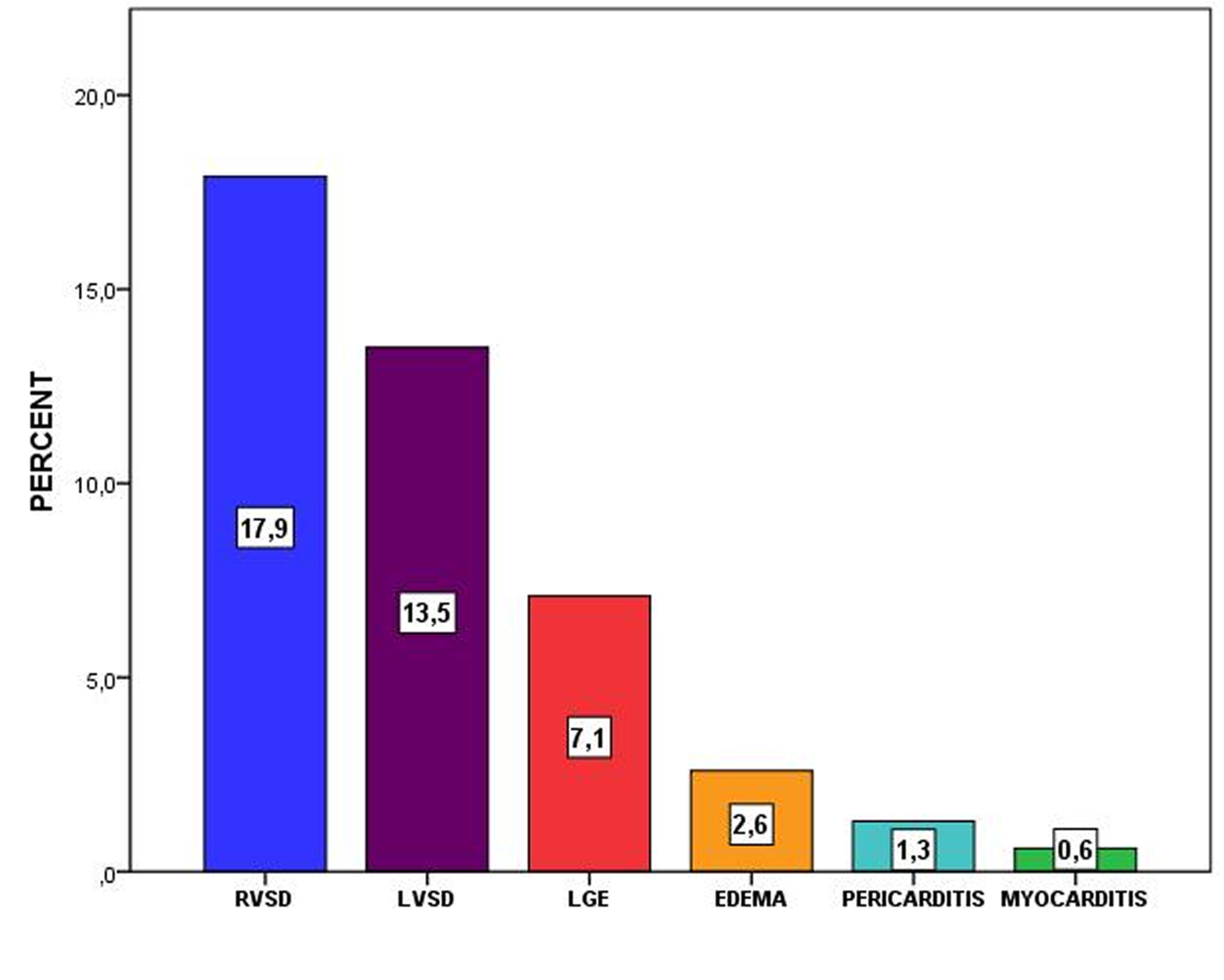
Fig.1 Findings in CMR. Components of CINV
Table 2: Clinical parameters of patients who presented CINV and those who had normal CMR.
|
Parameter |
NORMAL 111 (72.2%) |
CCOMP 45 (28.8%) |
P value |
|
Age mean ± SD |
47.5 ± 13.1 |
50.7 ± 13.7 |
0.18 |
|
|
|
|
|
|
Gender n (%) |
|
|
|
|
Male |
53 (60.9) |
34 (39.1) |
0.002 |
|
Female |
58 (84.1) |
11 (15.9) |
|
|
|
|
|
|
|
Body mass index mean ± SD |
28.4 ± 5.5 |
28.1± 4.7 |
0.71 |
|
|
|
|
|
|
HTN n (%) |
|
|
|
|
NO |
75 (73.5) |
27 (26.5) |
0.37 |
|
YES |
36 (66.7) |
18 (33.3) |
|
|
|
|
|
|
|
DBT n (%) |
|
|
|
|
NO |
97 (72.4) |
37 (27.6) |
0.40 |
|
YES |
14 (63.6) |
8 (36.4) |
|
|
Smoker n (%) |
|
|
|
|
NO |
77 (71.3) |
31 (28.7) |
0.95 |
|
YES |
34 (70.8) |
14 (29.2) |
|
|
|
|
|
|
|
COPD n (%) |
|
|
|
|
NO |
108 (71.5) |
43 (28.5) |
0.45 |
|
YES |
3 (60) |
2 (40) |
|
|
|
|
|
|
|
Previous CAD n (%) |
|
|
|
|
NO |
108 (71.5) |
43 (28.5) |
0.45 |
|
YES |
3 (60) |
2 (40) |
|
|
|
|
|
|
|
Previous stroke n (%) |
|
|
|
|
NO |
111 (72.1) |
43 (27.9) |
0.08 |
|
YES |
0 (0) |
2 (100) |
|
|
|
|
|
|
|
Charlson comorbidity score mean ± SD |
0.73 ± 1.2 |
1.2 ± 1.6 |
0.10 |
|
|
|
|
|
|
Admission due to COVID-19 n (%) |
|
|
|
|
NO |
77 (76.2) |
24 (23.8) |
0.04 |
|
YES |
34 (61.8) |
21 (38.2) |
|
|
|
|
|
|
|
Non-mild cases |
|
|
|
|
NO |
73 (79.3) |
19 (20.7) |
0.007 |
|
YES |
38 (59.4) |
26 (40.6) |
|
|
|
|
|
|
|
Days since discharge to CMR median (IQR) |
62 (43-84) |
54 (44-82) |
0.64 |
The location of LGE was subepicardial in 45.4% of cases, mesocardial in 36.4%, and subendocardial in 18.2%. Patients who presented LGE had a mean LVEF of 54.8% (SD 8.7%) and those who did not have LGE had 62.8% (p=0.0001). The mean RVEF in patients with no LGE was 57.8% (SD 6.6) and in those with LGE, it was 53.5% (p=0.04) (Figure 2).
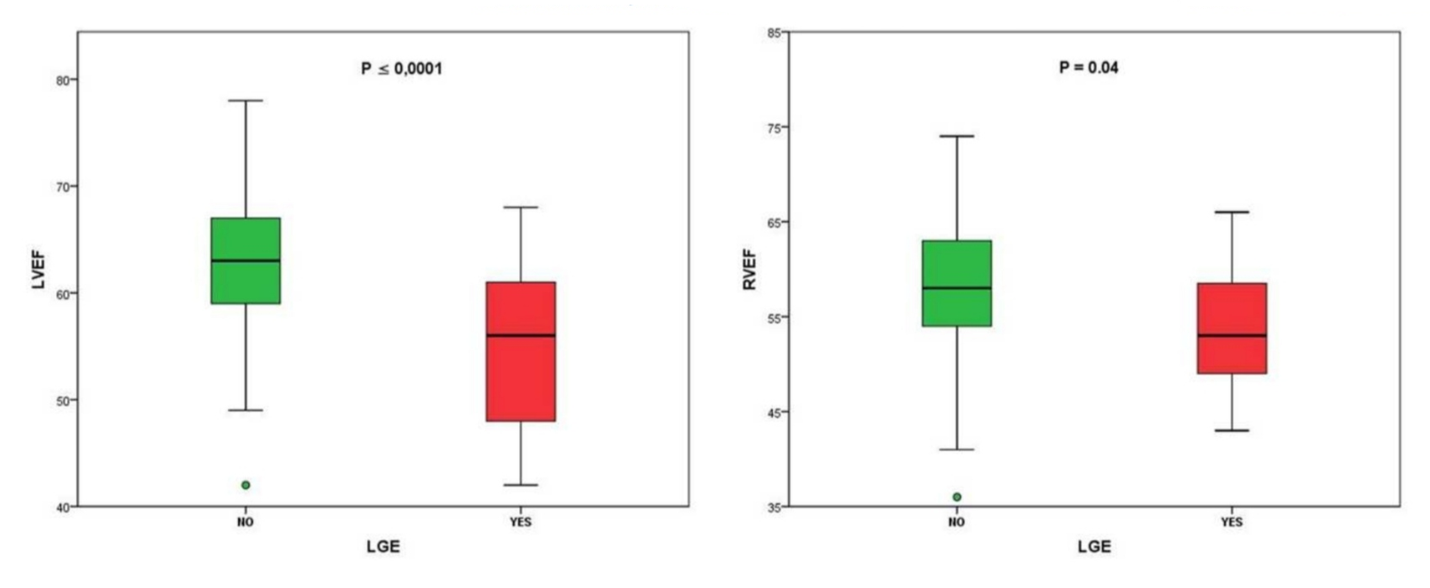 Fig. 2 LVEF and RVEF according to the presence of LGE.
Fig. 2 LVEF and RVEF according to the presence of LGE.
Discussion
In a cohort of 156 patients who recovered from COVID-19, in whom CMR was performed and who did not present troponin elevation at the time of performing the study, 28.8% CINV was found. RVSD and LVSD were the most frequently observed alterations, and in most of these, impairment was mild. In our study, the only preexisting condition that showed a significant difference in the presence of CINV was male sex, coinciding with data about a poor evolution in male patients with acute infection [16-17]. The patients who presented non mild cases and patients who required hospitalization presented higher prevalence of CINV. These data differ from the study by Puntmann et al, showing a higher prevalence of CINV (78%) and this finding is not related to previous comorbidities or the severity of acute clinical symptoms or hospitalization requirements due to COVID-19[11].
The most frequent alterations were those in relation to functionality and, to a lesser extent, those that may reflect a direct impact (inflammation, fibrosis, infection) on the heart. These alterations of functionality (with no tissue alterations) may be related to indirect CINV[18], i.e. dependent on multiple organ dysfunction, with myocardial oxygen supply-demand imbalance caused by the infection, which is generally observed in non-mild cases and is more frequent compromise than direct viral cardiac injury[19-21].
In our cohort, the prevalence of myocarditis was 0.65%, using the original Lake Louis diagnostic criteria[22]. These were updated in 2018 with the introduction of T1 and T2 mapping[23]. Only T2 mapping is better than the original Lake Louis criteria in terms of diagnostic sensitivity[24], and when both criteria are compared, the update only improves sensitivity with no impact on specificity. A myocarditis prevalence of 0% was observed in a series of 16 autopsies and 4.5% in 201 cases, with the latter being selected cases, which may entail an ever lower prevalence of myocarditis in the general population[25].
In our study, the presence of LGE, a prognostic marker[26], had a prevalence of 7.1%, which is substantially less than that observed in a recent study, where it was 30%. This also included patients with troponin elevation, and their values were more elevated in the patients who presented LGE[27]. Unlike this last study, in ours, the patients presenting LGE had less LVEF and RVEF (p=<0.001 and p=0.04, respectively) than patients who did not present them.
Limitations
This study presents the limitation of not having CMR data before the COVID-19 infection. Data are shown on a single CMR test of up to 3 months after the infection and an extended follow-up is essential to determine the progression or regression of cardiac compromise and its prognostic impact. We do not have T1 and T2 mapping parameters or extracellular volume estimation, which are part of the Lake Louis diagnostic criteria review. However, we did use diagnostic criteria for myocarditis that present a similar specificity.
Conclusion
Almost one third of patients recovered from COVID -19 with no troponin elevation, and cardiac alterations were found. These are more prevalent in patients of the male gender, who have required admission or who presented non-mild cases. Functional alterations (RVSD-LVSD) are most frequent. Myocarditis prevalence was very low. LGE is the most frequent tissue alteration and determinant for LVEF and RVEF.
Acknowledgements
To Maria Rosa Lombardo, Veronica Monzon and Liset Bringa for their valuable work. To JM Cullen Hospital and to everyone who has worked to treat this disease and lost their lives.
Declarations
Competing interests
The authors declare that they have no competing interests.
The authors declare that they have no sources of funding.Authors' contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by AC, LP, CP, LR, AB, JL,AB, MM, PDR, PG,MGV, SW and CF. DV performed cardiac resonance as a technician. LC performed the statistical and methodological analyzes. The first draft of the manuscript was written by AC, LP, LC and MM and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.