Clinical Research and Clinical Case Reports
OPEN ACCESS | Volume 6 - Issue 1 - 2025
ISSN No: 2836-2667 | Journal DOI: 10.61148/2836-2667/CRCCR
Zainab m. jawad Alkhirsan,1 Ali M Hussein Abdulhadi2*
1Jabir ibn hayyan University of Medical and Pharmaceutical Sciences, Faculty of Medicine, Najaf, Iraq.
2Jabir ibn hayyan University of Medical and Pharmaceutical Sciences, Faculty of Medicine, Najaf, Iraq.
*Corresponding authors: Ali M Hussein Abdulhadi, Jabir ibn hayyan University of Medical and Pharmaceutical Sciences, Faculty of Medicine, Najaf, Iraq.
Received Date: November 21, 2024
Accepted Date: November 28, 2024
Published Date: December 20, 2024
Citation: Zainab M. Alkhirsan J, Ali M Hussein Abdulhadi (2024). “Bacterial Toxins in Oncology: Exploring Their Potential as Cancer Therapeutics”. Clinical Research and Clinical Case Reports, 5(3); DOI:10.61148/2836-2667/CRCCR/88.
Copyright: © 2024 Ali M Hussein Abdulhadi, This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
One of the main causes of mortality in the globe is cancer. Because of the distinct pathophysiology of "solid tumors" and the predicted establishment of medication resistance, cancer treatment effectiveness is still a problem. There are drawbacks to conventional cancer therapies including immunotherapy, chemotherapy, and radiation therapy. Utilized either alone or in conjunction with conventional techniques, bacteriotherapy is a novel strategy that has demonstrated promise in tumor regression and metastasis reduction. The treatment of cancer with live, attenuated strains of bacteria, toxins, peptides, and bacteriocins is known as "bacteriotherapy." Additionally, they are frequently used as a vector to transport drugs, peptides, or genes to tumor sites. Remarkably, research has demonstrated that integrating them with conventional therapeutic approaches might enhance results. With the advent of genome editing, it is now feasible to produce a fresh strain of bacteria that fight cancer more effectively and with less adverse effects. This study aimed to provide an overview of current knowledge about the role of bacterial toxin in cancer therapy. This review focused on the latest developments in the field for different types of bacteria such as : "Corynebacterium diphtheria, Clostridium Spp Salmonella typhimurium (S. typhimurium)" and "Escherichia coli (E. coli)" and others species that mediated cancer therapy, including advances in the development of engineered bacteria for cancer treatment and further engineering techniques to enhance the delivery of therapeutic payloads. Lastly, we look over previous and current clinical research with microorganisms that target tumors.
Introduction:
Globally, cancer is a leading cause of illness and a financial burden. Due to global population increase and aging, we may anticipate 29 million cases by 2040, up from approximately 18 million cases in 2018. Although other cancers are also affected by bacteria, we use lung cancer as an example here. In 2017, lung cancer claimed more lives than the combined illnesses of breast, prostate, colorectal, and brain cancers . According to estimates, lung cancer accounted for 24% of cancer deaths in men and 15% in women in Europe in 2017, making it the top cause of cancer mortality for both sexes [1,2].
Utilize of bacteria and "bacterial products" in medicine began in the late 1800s when New York-based surgeon Dr. William Coley devised a method of inoculating cancer patients with bacterial mixes and/or bacterial toxins [1,2]. Many patients were discovered to be cured by bacterial injection, but because to variations in the toxins' formulations at the time—some of which were ineffective—these triumphs were difficult to repeat [3]. This approach, which involved using chemicals to stimulate the body's immune system to fight cancer, is today recognized as the first test of cancer immunotherapy, despite being later written off as untested by a large portion of the medical community and eventually eclipsed by radiation and chemotherapy.
Numerous microbial communities may be found in the human body, and the intricate relationships that exist between hosts and microbes are crucial to both human health and illness. Particularly, Certain bacteria residing in the human gastrointestinal tract, known as the gut microbiota, influence a range of metabolic and immune-mediated disorders, including obesity, malnutrition, and intestinal inflammatory diseases, as well as anticancer immunity [4]. The identification of these host-microbial interactions offers a way to modify the composition and activity of microorganisms and/or their byproducts in order to treat illnesses. Molecular biology and metabolic engineering concepts are employed in synthetic biology to create biological circuits with potential medical applications. Numerous instruments have been created for a variety of microorganisms, particularly chassis, enabling researchers to create disease-fighting mechanisms. It is possible to design engineered bacterial strains to detect and react to bodily environmental cues, such as those found in the tumor microenvironment (TME) [5,6].
2. History review of Using Bacteria in Cancer Therapy:
Patients with a Streptococcus pyogenes skin infection showed tumor regression, as noted independently by German doctors Busch and Fehleisen [7]. Separately, in 1893, sarcoma patient William Coley found that a patient had completely healed from an unintentional erysipelas infection. Using dead strains of Streptococcus pyogenes and Serrati marcescens, he observed tumor reduction in patients undergoing clinical trials for terminal malignancies. Later, Coley created a vaccination known as "Coley's toxin" from these " two bacterial species". This vaccine was widely utilized to treat a variety of tumors by simulating infection by causing fever, chills, and inflammation [8]. Coley's toxins did not become the standard of care in cancer therapy because of they were difficult to make or administer and had unfavorable side effects including fever. However, today's advancements in bacteria-based cancer therapy were made possible by the early effectiveness of Coley's toxins. When Morales, Eidinger, and Bruce utilized attenuated Mycobacterium bovis to treat bladder cancer in 1976, they effectively developed bacterial cancer treatment with Bacillus Calmette–Guerin (BCG) [9].
Other efforts have been made to treat cancer using genetically modified "bacterial toxins in combination with other treatments" such as (Salmonella, Clostridium, Lactobacilli, E. coli, Bifidobacterium, Pseudomonas, Streptococcus, Proteus, Caulobacter, and Listeria) and live bacteria (Streptococci and Clostridia) [8].
3.Bacterial therapy:
Subsequent to Coley's initial discoveries, research revealed that certain anaerobic bacterial species, particularly those within the genus Clostridium, proliferate in and consume oxygen-depleted cancerous tissue, yet perish upon exposure to the oxygenated regions of the tumour, thereby remaining non-harmful to surrounding healthy tissue [10]. These results gave rise to the justification for utilize the bacteria as oncolytic agents. But not all of the cancerous tissue is consumed by the bacteria, which is why chemotherapy medicines must be added to the therapy. Bacteria may therefore behave as chemotherapy sensitizers. To some degree, bacterial compounds such as lipopolysaccharides, or endotoxins, have already been investigated as potential cancer treatments. Tumor destruction can be achieved by the use of bacterial toxins, and cancer vaccines derived from these toxins can be developed [11].
It is possible to use bacteria as gene therapy vectors and as delivery systems for anticancer medications. Anaerobic bacteria's spores can be employed for the previously described techniques since they can only germinate, proliferate, and become active when they reach an oxygen-starved region of a tumor. Both bacterial gene-directed enzyme prodrug treatment and the use of genetically engineered bacteria for the targeted elimination of malignancies have showed promise.
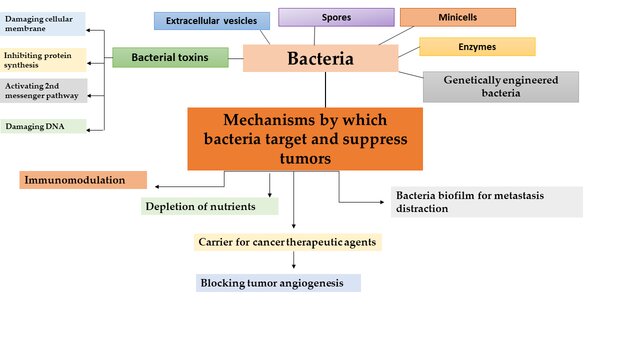
Figure (1): Various strategies in bacteria-based cancer therapy, along with the mechanisms through which bacteria and bacterial toxins target and inhibit tumors.
Utilize use of bacteria as carcinogens Live, attenuated, or genetically engineered nonpathogenic bacteria have started to be used as possible anticancer medicines. They can be used to carry tumoricidal chemicals or to provide direct tumoricidal effects. According to experimental investigation, Pathogenic species of anaerobic clostridia demonstrated a greater propensity for proliferation in the necrotic (anaerobic) regions of animal tumors compared to normal tissues. This led to tumor regression but was also associated with acute toxicity, and the majority of the animals either became sick or died [10,12].
This caused the attention to move to A non-pathogenic strain of Clostridium, such as 'M55,' has shown it does not produce significant tumor reduction. it was able to colonize anaerobic portions of the tumor after being administered intravenously [13]. Anaerobic bacterial species, such as lactobacilli, pathogenic clostridium, and bifidobacteria, have recently been tested for their capacity to proliferate in animal tumors used for experimentation. Clostridium novyi revealed significant anti-tumor effects; however, these tests also led to fatalities. After removing a gene that codes for a deadly toxin, an attenuated strain known as C. novyi-NT was created that showed promising outcomes but also generated toxicity. Therefore, traditional chemotherapy drugs such as dolastatin-10, mitomycin C, vinorelbine, and docetaxel were given in addition to C. novyi-NT spores. Although this tactic, called combination bacteriolytic treatment (COBALT), had notable anti-tumor effects, animal fatalities were still a possibility [14]. In clinical tumor models, C. novyi has been investigated alongside radiation, radioimmunotherapy, and additional chemotherapy [15,16].
The outcomes have shown that mixed multimodality techniques have the potential to be developed into future cancer therapeutics. Because of C. novyi-NT's clear capacity to break membranes, it has been used to improve the release of medications contained in liposomes within malignancies. Liposomase has been identified as the bacterial component responsible for the improved medication release. Studies in this area have been prompted by the remarkable removal of tumors in mice with big, established tumors when C. novyi-NT and a single dose of liposomal doxorubicin were used [17]. C. novyi-NT was utilized in conjunction with anti-microtubule medicines to enable medication delivery to the weakly vascularized sections of malignancies. Results showed that microtubule stabilizers like taxanes, docetaxel, and MAC-321, but not microtubule destabilizers like HTI-286 and vinorelbine, significantly decreased blood flow to tumors, expanding the hypoxic area that is favorable for spore germination [18].
The most effective bacterial agent to date, Bacillus Calmette-Guerin (BCG), is used only to treat superficial bladder cancer. A Salmonella typhimurium derivative strain called VNP20009 has recently been created for use in the treatment of cancer. The complete attenuation, achieved by preventing toxic shock in animal hosts, and dependence on external purine sources for survival, resulted from the deletion of two genes, msbB and purI. Because of this reliance, the organism can no longer reproduce in healthy tissues like the liver or spleen, but it may still develop in tumors where purine is present. This vector was able to target metastatic lesions in addition to demonstrating long-lasting effectiveness against a variety of experimental malignancies [19, 20]. Using Salmonella instead of Clostridium or Bifidobacterium has the benefit that it can thrive in both aerobic and anaerobic environments, which suggests that it can be effective against tiny tumors. In Phase 1 clinical studies, VNP20009 has been effectively studied in cancer patients. It's also conceivable that in the future, human clinical studies may assess additional live, attenuated bacteria, such Bifidobacterium and Clostridium. Salmonella choleraesuis, Vibrio cholerae, Listeria monocytogenes, and even Escherichia coli are among the novel bacterial strains being researched as anticancer agents [21].
4.Bacterial strains and derivatives for drug delivery carriers:
The medicinal properties of these species have been widely used to treat a variety of diseases, including cancers [22], gastrointestinal disorders like inflammatory bowel disease [22,23], diabetes [24], and obesity [22,25], because the special qualities of these species may be able to overcome the limitations of the current conventional therapies.
Bacterial genera evaluated in preliminary research and clinical therapy trials comprise Streptococcus [26], Clostridium [27,28], Bifidobacterium [29,30], Listeria [31-33], Escherichia [34], Lactobacillus [30], and Salmonella [35]. Previous to this, Streptococci and Clostridia have been tested in human trials of bacteria-based cancer therapy [36]. In recent times, bacterial species have undergone genetic engineering to enhance their safety and effectiveness in treating many malignancies and non-cancerous illnesses. Indeed, the distinct characteristics of these bacteria—such as their ability to produce therapeutic compounds and locate illness sites—have a significant bearing on the particular strategies used in bacteria-based therapeutics. While maintaining some of the inherent characteristics of their parent cells, such as immunological regulation and the capacity to load certain antigens and therapeutic payloads, bacterial derivatives offer a number of benefits over other bacteria. OMV and minicells' nanoscale diameters, which vary from 20 to 400 nm, are one of its advantages. Because of this characteristic, they are very biocompatible and simple for APCs to absorb. A Second, because their non-viability lowers the possibility of bacterial transmission and growth to non-target areas, BGs have higher safety profiles than live bacteria within their appropriate dose ranges [37].
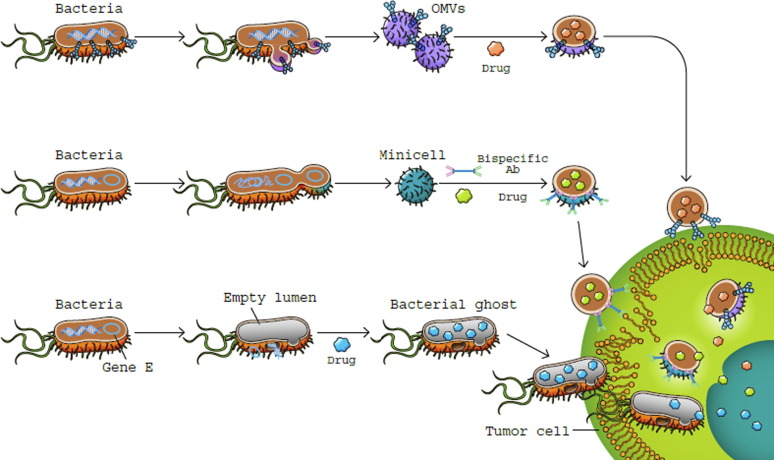
Figure (2): bacterial derivatives as a treatment for cancer. OMVs (outer membrane vesicles) produced by bacteria (top row). Gene-modified bacteria that express tumor-targeting moieties on their surface produce OMVs, which are drug-loaded anticancer microbes.
5.Bacterial toxins for cancer treatment:
To some extent, bacterial toxins have already been investigated as potential cancer treatments. The mechanisms that regulate cell division, apoptosis, and proliferation can be affected by bacterial toxins, which can also kill cells at low concentrations. These changes are linked to the development of cancer and have the potential to either promote aberrant cell behavior or obstruct regular cell functions.
Cell-cycle inhibitors, such cycle inhibiting factor (Cif) and cytolethal distending toxins (also known as CDTs), prevent mitosis and are believed to weaken the immune system by preventing lymphocytes from growing clonally. On the other hand, cell-cycle stimulators that impede cell differentiation and encourage cellular proliferation include the cytotoxic necrotizing factor (CNF) [38].
Cyclomodulins are a family of bacterial toxins that disrupt the eukaryotic cell cycle of their host. For instance, certain bacteria, including E. coli, emit CNF, a stimulant of the cell cycle. DNA replication is induced and the G1-S transition is triggered by CNF. However, there is no growth in the number of cells. Instead, the cells undergo multinucleation, possibly as a result of the toxin's capacity to obstruct apoptosis and cell differentiation [39, 40]. Gram-negative bacteria that include CDTs include Campylobacter jejuni and S. typhi, whereas enteropathogenic (EPEC) and enterohaemorrhagic (EHEC) E. coli contain Cif. Comparing the anti-tumor impact of toxins to conventional tumor treatment, the latter is likely associated with less adverse effects. Therefore, bacterial toxins alone or in combination with radiation or anti-cancer medications may improve the effectiveness of cancer treatment [41].
6. Types of Bacterial toxins
6.1 Diptheria Toxin:
In 1888, Roux and Yersin made the initial discovery of diphtheria toxin (DT) in Corynebacterium diphtheriae (42). A single-chain protein containing an enzymatic A domain (amino acids 1–193), a binding B domain (amino acids 482–535), and a translocation domain (amino acids 482-535) in the middle of the molecule, the DT gene is present on bacteriophages (43). On the surface of cells, DT binds to the precursor of heparin-binding epidermal growth factor-like growth factor (HB-EGF-EGF). On the plasma membrane, the HB-EGF precursor combines with CD9 and the proteoglycan heparan sulfate. Through its ability to inhibit heparin-binding epidermal growth factor, reduce tumor proliferation, lower angiogenesis, induce apoptosis, and function as an immunological adjuvant, CRM197, a mutant and benign form of diphtheria toxin, has demonstrated substantial anticancer capabilities. Moreover, this mutant form is used with traditional therapy, such doxorubicin, to lessen side effects and increase cytotoxicity (44). Another variation of this toxin that targets the tumor vascular endothelium and may aid in tumor regression in mice is called DTAT, a DT-based immunotoxin (45). The adrenocortical carcinoma cell lines H295R, colon cancer cell lines SW480, SW620, HCT116, CaCo-2, and HT-29, as well as gastrointestinal cancer cell lines, are all susceptible to the cytotoxic effects of diphtheria toxin (46, 47).
6.2 Clostridium difficile toxin:
Enterotoxin (TcdA) and cytotoxin (TcdB) are produced by Clostridium difficile, respectively (48). TcdB causes necrosis and apoptosis, reduces cell proliferation, and generates pro-inflammatory chemokines and cytokines in order to suppress cell proliferation (49). TcdB has potential applications as an anti-tumor vaccine or immunotherapy agent in the fight against cancer, supported by research demonstrating its high immunogenicity and ability to generate long-term anti-tumor immunity against various cancer cell lines, including CT26 colorectal cancer cell lines (50).
6.3 Clostridium perfringens Enterotoxin:
The toxin consists of a single polypeptide chain comprising 319 amino acids, with a molecular mass of 35 kDa (51). This pore-forming toxin induces lysis of epithelial cells by interacting with the transmembrane tight junction proteins claudin-3 and -4 (52). Large quantities of these two junction proteins have been detected in some human malignancies, such as ovarian, colon, and breast tumors (53). Through a number of mechanisms, including as pore creation in the cell membrane and binding to claudins, which results in rapid cell death, CPE has anticancer characteristics. Moreover, gene therapy typically uses the optimized CPE expressing vector (optCPE), A synthetic variant of CPE is designed to target the overexpression of the claudin-3 and/or -4 genes in human cancer. Furthermore, the disruption of the membrane leads to necrosis in claudin overexpressing cells and inhibits the proliferation of various human cancer cell lines, including HCT116, SW620, SW480, HT-29, and CaCo-2 for colon cancer, as well as HGC27, BGC823, MGC803, AGS, MKN45, and SGC7901 for gastric cancer (54).
6.4 Verotoxin 1:
Verotoxin 1 (VT1), sometimes referred to as Shiga toxin-1 (Stx-1) (HUS), is released by enterobacteriaceae groups that include hemolytic uremic syndrome (HUS), enterohemorrhagic E. coli (EHEC), and VT-producing E. coli (VTEC) (55). Inhibiting protein synthesis, proliferation, and cell cycle arrest in the S phase, VT1 binds the membrane receptor Gb3, overexpressed in many human cancer cell lines that are multidrug resistant (MDR) (56). VT- may be able to halt the cell cycle of colon cancer cell lines HCT116 (57).
6.5 Exotoxin A Pseudomonas aeruginosa:
Releases this 66 kDa toxin-containing substance. Through ADP-ribosylating elongation factor-2 (EF-2), exotoxin A inhibits protein synthesis and kills tumor cells (58). In PaCa2 pancreatic cancer cell lines, exotoxin A deimmunized in combination with human epidermal growth factor (EGF) and interleukin-4 (IL-4) has strong anti-tumor activity (59).
6.6 Immunotoxin Immunotoxins:
Are a cutting-edge method of treating cancer because they specifically target cells that have distinct antigens or surface receptors. A ligand, such as a growth factor (GF), monoclonal antibody (mAb), or antibody fragment, is affixed to a protein toxin to form immunotoxins.After binding of the ligand subunit to the target cell surface, the molecule is internalised, resulting in cell death due to the poison. Two bacterial toxins employed for targeting cancer cells are pseudomonas exotoxin and diphtheria toxin. These toxins are suitable for the production of recombinant single-chain or double-chain fusion toxins. Immunotoxins have been developed to target solid tumours and haematological malignancies by focussing on various growth factor receptors and antigens (60).
6.7 Escherichia coli toxins:
Two variants of E. Coli toxins belong to the shiga toxin (Stx) family: bacteriophages encode Stx1 (VT1, also referred to as Shiga-like toxin: SLT1) and Stx2 (VT2, SLT2). VT1 has been investigated for its anticancer properties due to its ability to bind to specific receptors on the surface of certain malignant tumour cells, leading to cell death by inhibiting protein synthesis. Select Therapeutics is developing a technique to express verotoxin receptors on human dendritic cells. Brain, testicular, breast, and ovarian cancers are the initial tumours to be prioritised. Verotoxin was administered to human astrocytoma tumour xenografts in nude mice (61). A single intratumoral injection of VT1 at a moderate dosage resulted in complete tumour remission in all treated subjects. Apoptotic processes were observed in both tumour and vascular cells within the treated xenograft. It was shown that verotoxin bound to tumor cells and blood vessels in sections of primary glioblastoma multiforme.
6.8 Fusion toxins that incorporate Pseudomonas exotoxin:
Fused with PE40, transforming growth factor- was one of the earliest fusion toxins that incorporate Pseudomonas exotoxin (PE). It worked well against tumor cells that expressed the epidermal growth factor receptor (EGFR). In clinical trials, it was investigated as an intravesical therapy for bladder cancer (62). This medication is being researched to help individuals with multiple myeloma clean their marrow before receiving an autologous bone marrow transplant.
6.9 Transferin:
-CRM 107 This is a genetic mutant of diphtheria toxin (CRM 107) that does not have native toxin binding combined with human transferin (TS). Glioblastoma has been treated with interstitial infusion, which crosses the blood-brain barrier (63). At least 50% of the patients had their tumor volume decreased. Clinical systemic toxicity was absent.
6.10 IL-4 fusion toxin: NBI-3001, an experimental medication developed by Neurocrine Biosciences in San Diego, CA, USA, combines interleukin-4 (IL-4) with an exotoxin derived from Pseudomonas bacteria. It binds to IL-4 receptors, which are found on malignant brain tumors but not on healthy brain cells, with high affinity when delivered directly into the tumor via a specialised catheter. Because of this, it can remove a significant amount of the tumor without harming healthy brain tissue. A phase I/II dose escalation, safety and efficacy research is presently being carried out in individuals with recurrent glioblastoma (64).
|
Toxin |
Origin |
Molecular weight |
GI Cancer Cell Line |
Other Human Cancer Cells/Cell Lines |
Ref |
|
Diphtheria toxin |
Corynebacteri um diphtheria |
60 kDa |
Adrenocortical carcinoma H295R, coloncancer (SW480,SW620, HCT116,CaCo-2 and HT-29), |
Glioblastomas (U118MG, U373MG, U87MG), cutaneous T cell lymphomas (CTCL), cervical adenocarcinoma (HeLa), breast cancer (MCF 7) |
(Lewis et al., 2017; Lutz et al., 2014; RK et al., 2000) |
|
TcdB |
Clostridium difficile |
270 Kda |
Colorectal cancer (CT26) |
Breast cancer (MDAMB-231) |
(Tuxiong et al., 2014; Yunli et al., 2018) |
|
CPE enterotoxin |
Clostridium perfringens |
35-kDa, (319 aa) |
Human colorectal cancer cell lines (SW480, SW620,HCT116, CaCo-2, and HT29), Human gastric cancer cell lines (SGC7901, MKN45, AGS, MGC803, BGC823, and HGC27) |
Human hepatocarcinoma (HepG2 cell, SK-HEP-1 cells), breast cancer (4T1), ovarian cancer, and prostate cancers |
(Zheng et al., 2017; Saeki et al., 2009; Pahle et al., 2017; Black et al., 2015) |
|
Exotoxin A |
Pseudomonas aeruginosa |
66 kDa |
Pancreatic cancer |
Melanomas (FEMX, Melmet-1, Melmet-5, Melmet44, MelRM, MM200), head and neck squamous carcinomas, Burkitt’s lymphoma (Daudi, CA46), leukemias (EHEB, MEC1), breast cancer (MCF-7, BT-20, CAMA1, SKBR-3). |
(Hemmat i et al., 2017; Bjorn et al., 1986) |
|
Verotoxin 1 |
E.coli |
70 kDa |
Human colorectal cancer cell lines (HCT116) |
ung cancer (A-549, PC14, and RERF-LC-AI), ovarian carcinoma, breast tumor cell lines (T47D, MCF-7) |
(Bhattac harjee et al., 2005) |
(Table 1): Some bacterial toxins have a lot of potential for cancer therapy described as follows:
7.Bacteria as immunotherapeutic agents:
Cancer immunotherapy is a new and promising treatment that has a lot of potential. The immunotherapeutic approach uses immune system activation to kill malignant cells since tumors are immunogenic. The capacity of malignancies to evade the immune system through tolerance development—despite their low immunogenicity and occasional body misinterpretation as self antigens—remains the main obstacle. Therefore, bacteria are used in one of the innovative immunotherapeutic techniques to increase the antigenicity of tumor cells [65]. S. typhimurium has been shown to infect cancerous cells both in vitro and in vivo, attenuating but still causing an inflammatory response. Successful invasion of melanoma cells by attenuated S. typhimurium has been shown; these cells can display bacterial antigenic determinants and serve as targets for T lymphocytes that are specific to Salmonella. Better results, however, were obtained when S. typhimurium vaccination was given to tumor-bearing animals prior to intratumoral Salmonella injection [66].
Attenuated strains of S. typhimurium that have undergone genetic engineering and produce murine cytokines have demonstrated the ability to modify immune responses to infections and impede the formation of melanomas in experiments. According to research, Salmonella organisms that encode IL2 are more effective in inhibiting the formation of tumors than their non-cytokine-expressing parental strain [67]. Salmonella and Listeria, two bacteria that have been exposed to tumor antigen DNA sequences, have developed protective immunity in animal models. It has been reported that oral administration of a xenogenic DNA vaccine expressing human tumor endothelial marker 8 (TEM8) carried by attenuated S. typhimurium induces a CD8 cytotoxic T-cell response specific to TEM8. The potential of antiangiogenesis immunotherapy is supported by the suppression of angiogenesiss in tumors, decreased tumor development, and protection of mice against fatal challenges against tumor cells [68]. It has been documented that C. novyi causes severe inflammation and leukocytosis. Moreover, inflammation's antitumor effects are now widely recognized. When C. novyi-NT spores are administered systemically, They eliminate adjacent cancer cells and induce an inflammatory response through the release of cytokines, including :IL-6, MIP-2, G-CSF, TIMP1, and KC, which draw inflammatory cells like neutrophils, monocytes, and lymphocytes.
By producing proteases, reactive oxygen species, and other degradative enzymes, the inflammatory response limits the bacterial infection and directly aids in the death of tumor cells. Lastly, it triggers a strong cellular immune response that eliminates any remaining tumor cells. A phase I clinical study combining an antimicrotubuli drug and C. novyi-NT spores has been started. Listeria monocytogenes is a facultative intracellular bacteria that has been employed as a vector for cancer vaccines due to its potent ability to trigger both innate and cell-mediated immunity. A regenerated Listeria monocytogenes vaccine strain (Lm-NP), which expresses nucleoprotein (NP) from influenza strain A/PR8/34, has shown significant therapeutic potential in preclinical settings by regressing the growth of various macroscopic tumors. The majority of tumor-bearing mice have shown successful recovery after treatment with another recombinant listerial strain, Lm-LLO-E7. Furthermore, Lm-LLO-E7 is presently undergoing clinical trials as a cancer immunotherapeutic for cervical cancer [69]. (In the syngeneic, aggressive mouse breast tumor model 4T1), an attenuated Listeria monocytogenes (LM)-based vaccination expressing truncated listeriolysin O (LLO) was shown to eradicate all metastases and almost the whole primary tumor [70].
Great effectiveness of a Listeria-based The growth of primary tumors and the spread of pulmonary metastases have been reported to be inhibited by a recombinant strain of attenuated S. typhimurium that expresses a gene encoding LIGHT, a cytokine known to promote tumor rejection, in a variety of mouse tumor models that use murine carcinoma cell lines in immunocompetent mice. Significant toxicity was avoided in the pursuit of antitumor activity [71]. The cell wall skeleton of Mycobacterium bovis Bacillus Calmette-Guérin (BCG-CWS) has proven to be an effective adjuvant for the immunotherapy of various cancer patients [72]. It has recently been shown that BCG/CWS induces autophagic cell death, which has a radiosensitizing impact on colon cancer cells. Studies conducted both in vivo and in vitro have demonstrated that BCG/CWS with ionizing radiation (IR) is a viable therapeutic approach for improving radiation treatment in colon cancer cells [73]. These results show that nonvirulent microorganisms have a promising future as cancer immunotherapeutic agents.
8.Bacterial spores:
Most of the anaerobic bacteria that have been studied so far are capable of forming so resistant spores that enable them to live even in environments that are abundant in oxygen, even though they are unable to grow or multiply there. However, the spores may germinate and the bacteria can flourish if they meet favorable circumstances, like the dead zones inside tumors, which makes them perfect to target malignancies. Without any systemic side effects, the spores of the genetically engineered strain C. novyi-NT, which is free of the deadly toxin, have demonstrated targeted activity. When C. histolyticum spores were injected intratumorally into mice, there was a noticeable lysis of the tumor tissues. Mice given intravenous injections of C. sporogenes spores showed the same behavior. Furthermore, in mice that received an intravenous infusion of bacteria, Clostridium was only found in tumors and not in normal tissues [74].
The reticuloendothelial system quickly removed the C. novyi-NT spores from the bloodstream, according to pharmacologic and toxicologic analysis. Even at high dosages, there was no evidence of clinical harm in rabbits or mice in good condition. On the other hand, toxicity in tumor-bearing animals seems to be correlated with both tumor size and spore dosage, as is the case with any bacterial infection [75]. Additionally, bacterial spores have been used as vectors for gene therapy, cytotoxic peptides, anticancer drugs, and therapeutic proteins.
9. Attenuation of bacterial virulence:
Immunity against bacterial vectors is the primary factor regulating the utilization of microorganisms as a medication delivery system [76]. Therefore, virulence needs to be reduced using genetic engineering techniques, which lead to the loss of key virulence genes and the creation of safe bacterial strains that retain their anticancer properties [77]. It was thought that S. typhimurium strain VNP20009, which had decreased septic shock (msbB) and purI genes depleted, had a satisfactory safety profile and tumor regressive qualities [78,79]. VNP20009 was therefore put to the test in phase I human safety testing.
VNP20009 was employed Utilised as a drug delivery system, this approach facilitates the expression of foreign proteins in cancer cells, such as endostatin, TNF-related apoptosis-inducing ligand (TRAIL), and chemokine ligand 21 (CCL21) [80,81]. Nevertheless, VNP20009 is not a viable option for delivering microRNA or short hairpin RNA (shRNA) as a plasmid carrier since it seldom ever releases plasmid into the cytoplasm of host cells. The cytoplasmic transcriptional regulator PhoP and the membrane-associated sensor kinase PhoQ were depleted, which led to a considerable reduction in intracellular bacterial survival. This allowed the mutant strain to transport a plasmid encoding shRNA to cancer cells with less harm [82].
Another non virulent strain of S. typhimurium, DppGpp, was obtained by deleting the relA and spoT genes. This strain lacks the ability to synthesize ppGpp, a signaling molecule that is necessary for the production of toxin genes. The DppGpp strain was nearly a virulent, as evidenced by its LD50 value, which was around 106 times greater than that of wild-type strains [83]. DppGpp Salmonella displays impaired intracellular growth and invasion because the production of the bacterial type III secretion system (T3SS), a needle-like structure utilized to invade eukaryotic cells, is ppGpp-dependent.
Because this strain is quickly removed by Macrophages in reticuloendothelial (RE) organs, where systemically inserted bacteria initially and temporarily localise, benefit from a deficit in intracellular survival. Through the activation of the toll-like receptor 4 (TLR4)/inflammasome and the generation of pro-inflammatory cytokines such as interleukin (IL)-1b, IL-18, and TNFa, the DppGpp strain exhibited strong anticancer activity. Bacteria are given a selection advantage by the ZnuABC zinc transporter, which allows them to flourish in low-zinc environments and increases the pathogenicity of certain Gram-negative bacteria.
Thus, testing an attenuated strain of S. typhimurium that was defective in ZnuABC for vaccination purposes in pigs and mice among other animal species revealed that this strain could be used in cancer therapy [85] and that it could elicit effective protection against enteric and systemic salmonellosis [84]. Both bacterial virulence and cytotoxicity were decreased as a result of LLO being depleted from L. monocytogenes [86]. Moreover, L. monocytogenes' capacity for intercellular dispersion and invasion was eliminated upon actA and inlAB gene deletion, respectively [87].. Certain nutrient-dependent mutations are a crucial tactic for producing less pathogenic strains. After systemically injecting the auxotrophic A1-R strain of Salmonella into tumor-bearing mice and isolating the bacteria again, it was shown that the bacteria tended to aggressively localize in tumors and demonstrated anticancer effects in a variety of animal tumor models. When L. monocytogenes' dal/dat locus was depleted, it became auxotrophic for D-alanine, which decreased the pathogenicity of the cell wall's constituents and increased the activity of cytotoxic T-lymphocytes. Exogenous adenine was necessary for bacterial mutant strains missing the purI and purD genes, which improved their capacity to actively multiply in purine-rich environments like tumor tissue [86].
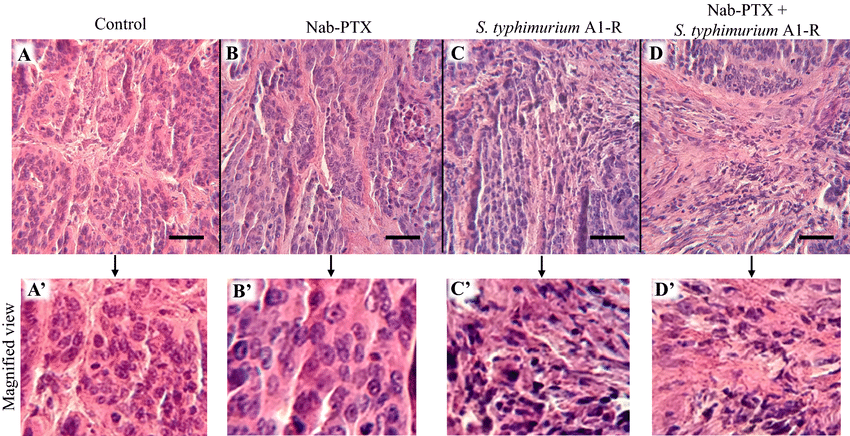
Figure (3): Treatment's impact on the histology of tumors. A Haematoxylin and Eosin (H&E) stain of the patient-derived orthotopic xenograft (PDOX) tumour, representing an untreated cervical carcinoma. H&E staining of the PDOX tumour subjected to nab-PTX treatment for cervical cancer. H&E staining of the PDOX tumour treated with S. typhimurium A1-R for cervical cancer. d H&E staining of the PDOX tumour related to cervical cancer treated with nab-PTX and S. typhimurium A1-R. A′–D′ Exhibit enlarged images. The scale bar measures 50 µm. Miyake et al. (2019).
10. Applications of bacteria-based therapy for disease management:
Preclinical research on immunotherapy for cancer Innate conditions seen in bacteria prevent the formation of tumors by either directly destroying tumor cells [90] or by modifying the host immune system [89]. Furthermore, the administration of immunomodulating drugs can be facilitated by designing microbes to have this immunotherapeutic effect. In a mouse tumor model, for instance, a strain of S. typhimurium that had been modified to release Vibrio vulnificus' flagellin B (FlaB) had a positive immunotherapeutic effect [91]. The TLR4 and TLR5 pathways are triggered by LPS and FlaB, which causes a large number of immune system cells, including neutrophils and monocytes/macrophages, to invade the tumor.
Furthermore, the production of tumor-suppressive cytokines and nitric oxide amplifies the therapeutic action of FlaB, which is released by colonizing Salmonella and causes M1-like macrophage polarization. Immunocheckpoint blockage was delivered using a probiotic system [92]. By lysing a release mechanism, engineered EcN 1917 released PD-L1 or CTLA-4 nanobodies, with bacteria demonstrating a strong anticancer impact in a murine tumor model. The populations of systemic T-cell memory and activating T-cells increased. Furthermore, the inclusion of GM-CSF, which indirectly attracts T-cells into the TME, improved the therapeutic effectiveness of this vector in the immunologically "cold" tumor.
Numerous cytokines have demonstrated anticancer effects, either directly through pro-apoptotic or anti-proliferative action or indirectly through inducing immune cell cytotoxicity against tumor cells [93]. Cytokine-expressing engineered bacteria have demonstrated improved anticancer activity on their own with less unfavorable systemic side effects. In multiple animal tumor models, for instance, S. typhimurium producing the TNF-family cytokine LIGHT [94] inhibited tumor development. Immunologic investigations demonstrated a notable infiltration of inflammatory cells in mouse tumours subjected to treatment with LIGHT-expressing bacteria. Animals treated with LIGHT-expressing bacteria exhibited intratumoral levels of CXCL-9 that were double those of animals treated with control bacteria, indicating a potential role of CXCL-9 in enhancing anticancer activity [95].The toxicity of these medicines toward non-tumor tissue limits their use, despite the fact that engineering bacteria has boosted their therapeutic anticancer efficacy. Bacteria administered intravenously often injure the liver and spleen when they first colonize these organs [96].
Therefore, to regulate gene expression in target tissue while preventing expression in normal tissue, an inducible promotor system was devised. For instance, exogenous doxycycline injection resulted in increased gene expression and a tumor reduction effect when S. typhimurium expressing ClyA was injected into animal tumors with a doxocycline-inducible gene expression system [97]. Similarly, after inducing Larabinose in a mouse tumor model, S. typhimurium that was designed to contain the anticancer protein Lasparaginase and a L-arabinose-inducible gene expression system [98] shown improved anticancer activity. Furthermore, sophisticated inducible systems that permit reactions to changes in cellular density (quorum sensing) [99], tissue hypoxia [100], and acidic pH [101] have demonstrated anticancer effects without requiring the annoyance of recurrent exogenous inducer injections.
Uses in medicine A patient with an erysipelas infection (Streptococcus pyogenes) had a neck sarcoma that was cleared, according to a report by Dr. Coley in 1891 [101]. Many clinical trials have examined bacteria-based cancer treatments to date. A clinical experiment including 12 cancer patients evaluated the safety and effectiveness of the mixed bacterial vaccine (MBV, Coley's toxins), which is made up of a combination of heat-killed S. pyogenes and Serratia marcescens [102]. Ten of the 12 patients had elevated body temperatures together with enhanced expression of immunoregulatory cytokines. A partial tumor response was seen in one patient who had metastatic bladder cancer; this response was connected with higher cytokine concentrations and fever generated by MBV.
This trial revealed that MBV has immunomodulatory properties and can operate as a powerful cancer vaccine. S. typhimurium strains have been evaluated in many phase I studies. For instance, a phase I study using S. typhimurium VNP20009 in patients with metastatic melanoma discovered that all subjects received the intravenous administration of the bacteria's maximum permissible dosage during a 30-minute period without risk [103]. However, none of the individuals who underwent testing showed objective tumor shrinkage; instead, they all had disease progression. Only one patient showed bacterial colonization of tumor tissue. Four patients underwent bacterial infusion for four hours in order to improve the transport of S. typhimurium VNP20009 to the tumor [104]. Despite the safe infusion of the bacterium, no objective clinical reaction was observed in any of the patients, and no salmonella was found in blood samples collected more than 6 hours after the infusion began.
The E. coli CD gene, which codes for an enzyme that changes the prodrug 5-FC into the cytotoxic agent 5-FU [105], was transferred into S. typhimurium VNP20009 in a different phase I clinical study to boost the drug's therapeutic impact. Three patients had repeated cycles of 5-FC plus an intratumoral injection of bacteria (two with esophageal cancer and one with refractory head and neck cancer). Intratumoral bacterial colonization and 5-FC to 5-FU conversion were seen in two of these patients. Compared to plasma, the concentration of 5-FU in tumors was three times greater. However, analysis of the clinical responses revealed that two patients had illness progression and one patient had stable disease. The experiment demonstrated that Salmonella has potential as a drug delivery vector for cancer treatment, notwithstanding the unimpressive clinical results.
Clinical experiments using L. monocytogenes platforms have yielded encouraging outcomes [106]. Three phase II clinical studies with different strains of Listeria have been finished thus far. A phase II multicenter trial, for instance, evaluated an engineered strain of L. monocytogenes (CRS-207) that secretes mesothelin, increasing T-cell production and anticancer activity, in ninety patients with metastatic pancreatic adenocarcinoma [107]. The combination of irradiation GM-CSF-secreting allogeneic pancreatic ductal adenocarcinoma cells (GVAX), low-dose cyclophosphamide (Cy), and CRS-207 improved patient survival when compared to CRS-207 alone. However, Cy/GVAX with CRS-207 did not increase survival in patients with metastatic pancreatic cancer, according to a second trial that examined CRS-207 alone, Cy/GVAX plus CRS-207, and conventional treatment [108].
An further experiment revealed that transgenic L. monocytogenes (ADXS-11–001) immunotherapy was acceptable in 50 patients with platinum-refractory cervical cancer [109]. Of these patients, 39 had tumor responses assessed; only one had a partial response, five had stable illness, and the remaining 32 had progressing disease. The therapeutic effectiveness of this immunotherapy in patients with cervical cancer will be assessed in a phase III clinical study [110].
|
Type of bacteria |
Indication |
Phase |
Route of administration |
Year |
Number of enrolled patients |
|
Mixed bacterial vaccine |
Malignant tumor |
1 |
Subcutaneous |
2013 |
17 |
|
Salmonella typhimurium |
Unresectable hepatic tumor |
1 |
Oral |
2014 |
22 |
|
S. typhimurium (VNP20009) |
Advanced solid tumor |
1 |
Intravenous |
2002 |
45 |
|
S. typhimurium-IL2 (Saltikva) |
Metastatic pancreatic cancer |
2 |
oral |
ongoing |
NA not applicable |
|
Listeria monocytogenes (CRS207) |
Metastatic pancreatic cancer |
2 |
Intravenous |
2017 |
93 |
|
L. monocytogenes (CRS-207) |
Malignant pleural mesothelioma |
1 |
Intravenous |
2019 |
60 |
|
L. monocytogenes (ADXS-11-001) |
Cervical carcinoma |
2 |
Intravenous |
2018 |
54 |
|
L. monocytogenes (ADU-623) |
Brain tumor |
1 |
Intravenous |
2018 |
11 |
|
L. monocytogenes (JNJ64041809) |
Castration-resistant prostate cancer |
1 |
Intravenous |
2018 |
26 |
|
L. monocytogenes (CRS-207) |
Metastatic pancreatic cancer |
2 |
Intravenous |
2016 |
303 |
|
Clostridium novyi-NT |
Solid tumor |
1 |
Intratumoral |
2017 |
24 |
|
13 species of probiotics |
Breast cancer |
NA |
oral |
2020 |
7 |
|
Fecal microbiota |
Metastatic mesothelioma |
1 |
FMT fecal microbiota transplant. |
2018 |
1 |
Table (2): Some clinical trials with bacteria-based cancer therapy, (Knag et al ,2022).
11.Challenges in Bacterial Cancer Therapy:
As a multifactorial illness, cancer may be treated by using bacteria as a vector for therapeutic cargo or as an immune-stimulating agent (111). But one big problem with bacteria-mediated cancer treatment is toxicity. While greater dosages can be harmful and have negative consequences, lower levels can change the effectiveness of therapy (112). Therefore, it's important to establish a balance between the trial subject's benefit and safety (113). Tumor architecture in humans and preclinical animal models differ, which may have an impact on the proliferation and penetration of bacteria within the tumor (114). Consequently, it is necessary to optimize the delivery route and dosage. Moreover, therapy failure might result from the immune system eliminating microorganisms before they reach the tumor location (115).
Furthermore, any changes to the microorganisms may lead to exacerbated infections and therapeutic loss 51 | Page. Consider the difficulties associated with magnetotactic bacteria. One approach involves increasing the production of MTB and exploring synthesis methods to modify the magnetic properties of MTB through the manipulation of the magnetosome biomineralisation process. To address the challenges of scaling up MTB production, alternative strategies involve importing and expressing the genes required for magnetosome formation in other bacterial species that are more amenable to large-scale growth and culture (116). Altering the magnetosome's size, shape, and content throughout the biomineralization process is a another method to change the magnetic sensitivity of MTB.Still, according to Fedez et al. (2020), we are far from having a good grasp on the problem (117).