Clinical Research and Clinical Case Reports
OPEN ACCESS | Volume 7 - Issue 1 - 2026
ISSN No: 2836-2667 | Journal DOI: 10.61148/2836-2667/CRCCR
Ziadi Arwa*, Khadhar Yassine, Derbel Bilel, Miri Rim, Ziadi Jalel, Ben Mrad Melek, Denguir Raouf
Cardiovascular surgery department, La Rabta hospital, Tunis, Tunisia.
*Corresponding author: Ziadi Arwa, Cardiovascular surgery department, La Rabta hospital, Tunis, Tunisia.
Received: December 01, 2025 | Accepted: January 15, 2026 | Published: January 24, 2026
Citation: Arwa Z, Yassine K, Bilel D, Rim M, Jalel Z, Ben M Melek, Raouf D, (2026). “Aortobronchial Fistulas: Endovascular and Hybrid Aortic Repair Strategies — A Case Series ”. Clinical Research and Clinical Case Reports, 7(1); DOI: 10.61148/2836-2667/CRCCR/098.
Copyright: © 2026 Ziadi Arwa. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
An aorto-bronchial fistula is an exceptionally rare and severe condition that can be fatal without prompt emergency intervention [1]. Treatment options encompass both surgical and endovascular approaches, each presenting distinct benefits and drawbacks. While endovascular repair of the descending thoracic aorta has become a feasible treatment, its effectiveness for managing aorto-bronchial fistulas remains inadequately studied. This report details three atypical cases of aorto-bronchial fistula, where the condition initially presented as hemoptysis in all patients.
aorto-bronchial fistula
An aorto-bronchial fistula is an exceptionally rare and severe condition that can be fatal without prompt emergency intervention [1]. Treatment options encompass both surgical and endovascular approaches, each presenting distinct benefits and drawbacks. While endovascular repair of the descending thoracic aorta has become a feasible treatment, its effectiveness for managing aorto-bronchial fistulas remains inadequately studied. This report details two atypical cases of aorto-bronchial fistula, where the condition initially presented as hemoptysis in all patients.
Case 1:
A 71-year-old man with locally advanced squamous cell carcinoma of the lung was transferred to our institution because of intermittent massive hemoptysis over the preceding 6 weeks. He previously underwent a Chest computed tomography (CT) with intravenous contrast enhancement that disclosed a massive extravasation at the arterial phase due to a breach in the medial wall of the descending aorta, which is encased by the pulmonary tumor, along with an intra-alveolar hemorrhage in the left lung.
Initial clinical examination revealed tachypnea. He was hemodynamically unstable and severely hypotensive. Arterial blood gases showed severe hypoxemia and hypercapnia.
Endotracheal intubation was immediately performed in tandem with cardiovascular pharmacologic support.
The patient was immediately transferred to the angiography suite, where a 26 × 26 × 160mm stent graft was inserted into the descending aorta via a right femoral approach. The site of the aortobronchial fistula was entirely excluded.
Unfortunately, the patient died intraoperatively due to cardiogenic shock.
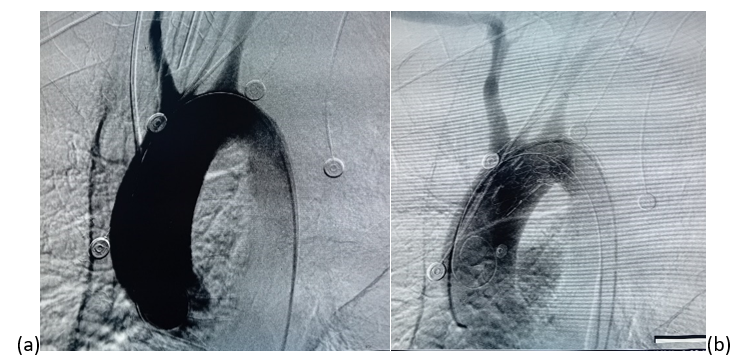
(a) Before stentgraft deployment, showing contrast extravasation indicating the presumed site of the malignant aortoesophageal fistula. (b) Completion angiogram after successful deployment 26 × 26 × 160mm stent graft with sealing of the aortobronchial fistula.
Case 2:
A 65-year-old woman with no previous medical history, presented at our emergency department with intermettent hemoptysis and chest pain radiating to the back for the past 3 weeks, with worsening over the last 48 hours.
A chest CT angiography was performed, revealing a 30 mm saccular aneurysm displacing the left lower lobe branch, along with an aortobronchial fistula.
Upon admission, the patient was afebrile and did not exhibit any signs of dyspnea. Her blood pressure and pulse were within normal ranges. Laboratory tests indicated anemia, with a hemoglobin level of 9 g/dL.
The patient underwent endovascular stenting of the aorta just distal to the origin of the left subclavian artery with a 36 × 28 × 200mm stent graft. The left subclavian artery was covered to secure an adequate proximal landing zone. Completion angiography was performed to assess accurate placement and exclusion of the aortic aneurysm.
The patient's recovery was uneventful, she has not had any further episodes of hemoptysis with no septic complication or need for an endobronchial stent.At the 6 month follow-up, her condition was good, and the CT scans revealed a satisfactory result.
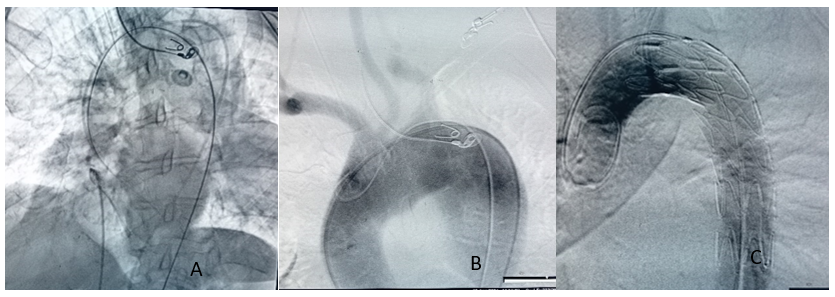
(A,B,C) An aortic stent-graft was deployed to cover the presumed site of the malignant aortobrochial fistula
Case 3 :
An 80-year-old woman with a history of diabetes and a pacemaker implantation presented to our emergency department with severe hemoptysis. Her hemodynamic status remained stable. A CT scan revealed a saccular aneurysm of the aortic arch, measuring 40 mm in diameter, with rupture into the left bronchus. The aortic arch itself had a diameter of 30 mm. There was no suitable landing zone, as the distance from the aneurysm to the innominate artery was only 2 mm. Given these findings, we opted for an urgent hybrid surgical intervention, with a two-part procedure strategy.
The first stage involved a surgical procedure, beginning with a sternotomy. We performed total supra-aortic debranching, followed by a bifurcated graft bypass from the ascending aorta to the innominate and left carotid arteries. Proximal anastomosis was achieved via lateral clamping of the ascending aorta. The second stage was the endovascular procedure and took place after chest closure. We placed a 38x100 mm endoprosthesis in the aortic arch, covering the origins of all supra-aortic trunks. The landing zone included the origin of the innominate artery (zone 0). The procedure was conducted using two femoral access points, and the final angiography was satisfactory.
Postoperatively, the patient initially showed favorable progress. Plans for an endoscopic closure of the aortobronchial fistula were made, but she developed sepsis, with a fever of 40°C and a severe left lung infection, requiring catecholamine support. Despite intravenous antibiotic treatment, the patient passed away on the eighth postoperative day.
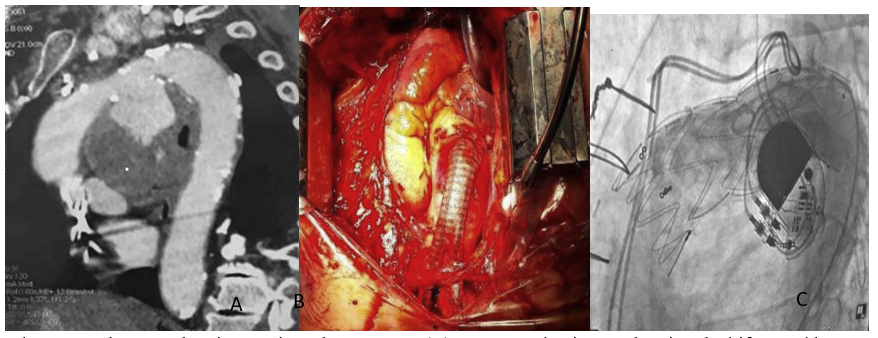
(A ) Computed tomography scan showing aortic arch aneurysm (B) Per operative image showing the bifurcated bypass grafting (C) Final angiography: endoprosthesis covering origins of all supra-aortic trunks.
Discussion:
Aortobronchial fistulas (ABFs) can arise from various pathological conditions affecting the descending thoracic aorta (DTA), such as atherosclerotic aneurysms, para-anastomotic pseudoaneurysms resulting from prior open surgeries, and mycotic aneurysms [2,3]. They typically present with significant or intermittent hemoptysis, making early detection and diagnosis crucial. If untreated, these fistulas can expand, leading to severe hemoptysis and potentially life-threatening hemorrhage.
Elective open surgery on the thoracic aorta carries a high risk of mortality and morbidity, especially since many results come from specialized centers of excellence[4] . With reported surgical mortality rates ranging from 18% to 24% and notable postoperative morbidity [5,6]. These risks are further higher in patients with unstable hemodynamic and respiratory conditions, as with ours.
Choosing the appropriate treatment for these patients is a dilemma. Since ,TEVAR is less invasive, as demonstrated by various comparative studies[7] , it is increasingly favored over open surgery.
This benefit is particularly significant in acute ABF cases, as confirmed by the lower perioperative complication rates reported in numerous reviews [1,5,8–10].
For our patient who died peropertively, he was referred from another hospital, with a significant time delay. He was severely hypotensive and hypoxemic. This underlines the need for immediate treatment.
While endovascular stenting is generally simpler, faster, and safer for unstable patients compared to surgery, it does carry risks such as leakage and migration[11].
Another concern is that the stent might be exposed to a contaminated environment. However, in the case of our second patient, the stent quickly became isolated from the bronchial lumen with no documented infections.Unfortunately , this wasn’t the case of our third patient who did not survive, succumbing to septic shock. We think that this complication occurred because of the rupture in the pulmonary bronchus.
Conclusion:
Based on our limited experience, we consider endovascular stenting as a safe and minimally invasive option for treating aortobronchial fistulas in unstable or at high-risk patients. However, several important issues remain unresolved, such as the long-term durability of the stent grafts. Further research and ongoing monitoring of graft performance are essential to better define the role of endovascular treatment in the future.