Clinical Case Reports and Clinical Study
OPEN ACCESS | Volume 12 - Issue 5 - 2025
ISSN No: 2766-8614 | Journal DOI: 10.61148/2766-8614/JCCRCS
Aksam Yassin1,3*, Anas Albudairat1, Hasan Abdallah1, Hatem Kamkoum1, Raidh Talib Alzubaidi1,2, Bassam Albaba4 and Abdulla Al-Ansari1,2
1Hamad Medical Corporation, Aisha Al Attiyya Hospital, Andrology & Men’s Health Unit, Qatar.
2Weill Cornell Medical School NY, Qatar.
3Dresden International University, Preventive Medicine Program, Germany.
4Sharjah University, Medicine & Cardiology, United Arab Emirates.
*Corresponding author: Aksam Yassin, Hamad Medical Corporation, Aisha Al Attiyya Hospital, Andrology & Men’s Health Unit, Qatar, Weill Cornell Medical School NY, Qatar, Dresden International University, Preventive Medicine Program, Dresden Germany.
Received: May 01, 2025
Accepted: May 06, 2025
Published: May 09, 2025
Citation: Aksam Yassin, Anas Albudairat, Hasan Abdallah, Hatem Kamkoum, Raidh Talib Alzubaidi, Bassam Albaba and Abdulla Al Ansari. (2025) “Imaging Evidence for Improving Erectile Function under Testosterone Therapy (TTh)” Clinical Case Reports and Clinical Study, 12(2); DOI: 10.61148/2766-8614/JCCRCS/206.
Copyright: © 2025 Aksam Yassin. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The main effect of testosterone was long-time assumed to be on sexual interest and, indirectly, on erectile function. Newer insights demonstrate that testosterone deficiency impairs the anatomical, ultrastructural, biological and physiological/functional substrate of penile erection, which can be, at least in part, restored by normalization of plasma testosterone levels. This is a report on a 56-year-old man suffering from diabetes mellitus type II and metabolic syndrome, who had complaints of a severe erectile dysfunction because of venous leakage, confirmed by pharmaco-cavernosography. He was also testosterone deficient (1.8 ng ml−1). Upon testosterone administration his erectile function improved dramatically. Repeated cavernosography no longer showed venous leakage.
Introduction and Background
Clinical issues
The powerful effects that testosterone exerts on a man's sexual functioning are impressively demonstrated by the physical and mental changes associated with pubertal development and by testosterone replacement in cases of male hypogonadism. Only in the 1980s, did the effects of testosterone on a man's sexual functioning became better understood. It appeared from these first studies that sexual interest is particularly stimulated by androgens. The effects of androgens on erectile function, as they emerged from these studies, were somewhat unexpected. It appeared that sleep-related erections were distinctly androgen dependent, whereas erections in response to erotic (e.g. visual or tactile) stimuli were substantially less dependent on circulating androgens. This discrepancy in androgen dependence between sleep-related erections and erections following explicit sexual stimuli led to the hypothesis that the two types of erections were subserved by different neural circuitries. The original studies, however, monitored only penile circumference but not stiffness, and later observations showed that androgens do affect penile responses to erotic stimuli regarding the duration of response, maximal degree of rigidity and speed of detumescence.
Several studies in hypogonadal men, with a broad range of ages, receiving testosterone replacement indicated that plasma testosterone in the low range of reference values (or even slightly below that range) sufficed to restore sexual functioning. Several recent studies indeed confirmed that testosterone administration as a single treatment of erectile dysfunction may be sufficient in up to 56% of cases (Yassin et al., 2003; Greenstein et al., 2005; Yassin, 2005a). Despite the initial euphoria of a panacea for erective dysfunction (ED), and the simplicity and safety of the therapy of ED with phosphodiesterase type 5 (PDE 5) inhibitors, it is not successful in all men to whom it is prescribed. Approximately 50% of patients discontinued treatment for a few reasons. Among these reasons testosterone deficiency is emerging as a significant factor. Several clinical studies now show that administration of testosterone improves the therapeutical response to PDE-5 inhibitors considerably (Aversa et al., 2003; Shabsigh, 2004; Yassin, 2005a). Another important finding is that maintenance of sexual functions in elderly men requires higher circulating levels of testosterone than younger men in whom it was found that low normal testosterone levels sufficed.
It has been hypothesized that the threshold for the biological actions of testosterone might be higher in elderly men compared with young men. Their hypothesis was recently convincingly experimentally confirmed, showing that in elderly men libido and erectile function respond only to higher levels of circulating testosterone than in younger men.
Basic research
The most revolutionary insight is, however, that testosterone has profound effects on tissues of the penis involved in the mechanism of erection and that testosterone deficiency affects the erection at the penile cellular level and impairs the anatomical ultrastructural, biological and physiological/functional substrate of erectile capacity, reversible upon androgen replacement. This data comes mainly from animal experimentation, but a number of studies support their relevance for humans as well. There are androgen receptors in the human corpus cavernosum. Aversa et al. (2003) demonstrated that the arterial inflow into the penis is improved by androgen administration. Foresta et al. (2004) have documented that nocturnal penile tumescence, arterial cavernous inflow and visually stimulated erection normalized upon administration of testosterone restoring plasma testosterone to normal.
It is established that the expression of nitric oxide (NO) synthesis (Burnett, 2004) is regulated by androgens. Several studies show that androgens play a critical role in restoring and maintaining the penile trabecular smooth muscle structure and function (Traish et al., 1999, 2003; Yassin, 2005b) as well as regulating the cell apoptosis (Shabsigh, 1997). Extra-cellular matrix and the fibro-elastic properties – in biology and microanatomy – are also regulated by androgens. Even the ‘healthy’ structure of the tunica albuginea is androgen dependent (Shen et al., 2003).
Testosterone plays a key role in the central and peripheral modulation of erectile function (Foresta et al., 2004). New research in the laboratory and in humans is shaping a refinement of the role of testosterone therapy in ED. Testosterone deficiency induces both biological and structural/functional changes in the trabecular cavernoma tissues. Adipocyte accumulation in penile subtunic area of the corpus cavernosum in orchiectomized rabbit emphasized the potential mechanism for Veno-occlusive dysfunction in androgen deficiency (Traish et al., 2005).
Most studies trying to elucidate the mechanism of action of androgens on penile erectile structures have been performed in laboratory animals. These observations suggest that androgens are necessary to maintain the integrity of the anatomical structures of the penile erectile tissue and, further, that androgens are significant in the biochemical mechanisms subserving penile erection. It remains to be demonstrated whether these mechanisms operate also in the human species, but this information is certainly of importance to guide further study in the human.
In a rat model, Shen et al. (2003) demonstrated that androgen deprivation leads to loss of elastic fibers in the tunica albuginea and of smooth muscle fibers in the corpus cavernosum, which were replaced by collagenous fibers in both structures. Similar findings were reported in the rabbit by Traish et al. (1999) noting a reduced trabecular smooth muscle content, reversible upon androgen replacement.
It is now clear that mesenchymal pluripotent cells follow a myogenic lineage with normal testosterone levels or, alternatively, an adipogenic lineage with low levels of testosterone. Traish et al. (2005) demonstrated a similar mechanism in the rabbit if testosterone levels are low, with an accumulation of fat containing cells in the subtunic region of the corpus cavernosum thus reducing the elasticity of the tunica albuginea. This study confirmed also that androgen deprivation leads to loss of trabecular smooth muscle and increase of connective tissue fibres.
In the study of Traish et al. (1999), it was also found that intracavernosal pressure, expression of alpha1-adrenergic receptor and PDE type 5 activity were clearly dependent on androgen, which was not the case for neuronal nitric oxide synthase protein expression. In a follow-up on this study Traish et al. (2003) demonstrated that even a 50% reduction in circulating testosterone reduced intracavernosal blood pressure, which was not enhanced by administration of the PDE-5-inhibitor vardenafil. The dependence of PDE type 5 on androgens in the muscular and endothelial compartment of the corpus cavernosum was confirmed in the rat but also in human tissue by Morelli et al. (2004).
Case report
Against the background of these new insights into androgen action on anatomical and physiological substrate of penile erection, we report a case in whom an impressive improvement of venous leakage was observed upon treatment with testosterone. A man, aged 56 years suffering from diabetes mellitus type II and metabolic syndrome, had complaints of severe erectile dysfunction since 3.5years. Upon diagnostic evaluation the patient received an intracavernosal injection of 20 μg AlprostadilTM, which did not produce a sufficient degree of erectile response. The suspected diagnosis of venous leakage was confirmed by penile pharmaco-cavernosography by intracavernosal injection of 20 μg Alprostadil, followed by injection of 60–80 ml contrast media Omnipaque 150 (Schering AG, Berlin, Germany; Fig. 1). Plasma testosterone was in the hypogonadal range (1.8 ng ml−1; normal range 4.0–8.6 ng ml−1). The latter finding led to treatment with testosterone. The patient received a long-acting testosterone preparation (testosterone undecanoate, (Nebido®), 1000 mg, with repeated administration after 6 weeks and thereafter every 12 weeks, following recommendations in the literature.
Figure 1: Cavernosography at baseline shows three types of venous leakage: in subtunical venules, superficial and profound venes.
The patient noted a significant improvement in his erectile function after approximately 9 weeks. In his sexual desire domain, there was an increase from four to eight and in the erectile function domain from 10 to 24. Particularly, the improvement in erectile function was impressive and the patient agreed to undergo a second penile cavernosography 16 weeks after start of androgen treatment. To our surprise, there was no evidence any more of venous leakage (Fig 2).
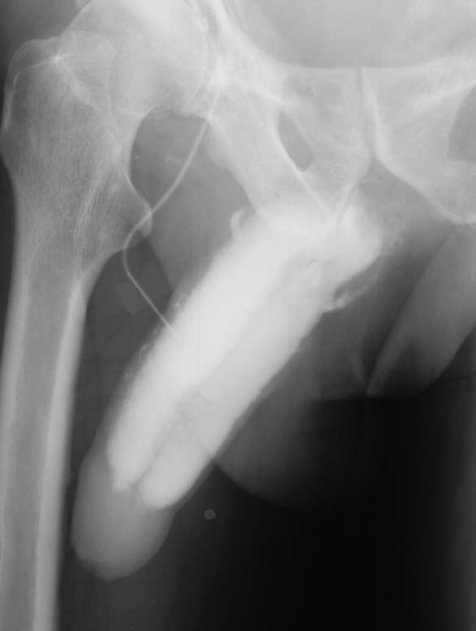
Figure 2: Cavernosography after 18 weeks of testosterone treatment with no evidence of venous leakage.
Discussion
A case report does not have the statistical power to reliably demonstrate the relationship between an intervention and the outcome of the intervention. It has the function to alert clinicians to potential treatment modalities hitherto unsuspected. We plan to replicate this intervention into a larger number of patients and stimulate other investigators to research this potentially beneficial treatment. Although success exceeded our expectations, the observation is consistent with the new insights that testosterone deficiency impairs the anatomical and physiological substrate of penile erection, which, fortunately, can be, at least in part, be restored by normalization of plasma testosterone levels. The time course between start of testosterone treatment and improvement, as reported by our patient, in clinical expression, was 9 weeks, and confirmed in the cavernosography after 18 weeks. If indeed the effects of testosterone on restoration of venous leakage could be confirmed in larger scale studies, this would be a major asset as venous leakage is a common cause of severe erectile dysfunction and was an absolute indication for penile implants in these cases (Elhanbly et al., 2002).
A somewhat unexpected view on a possible mechanism of the beneficial effects of testosterone in the reported patient is his potential endocrine imbalance. Elderly men with diabetes have often lowered plasma testosterone while their estradiol levels are relatively high, resulting in an estrogen/androgen imbalance. A recent study hypothesized that the estrogen dominance in elderly and/or diabetic men could affect vascular tone of the penile blood vessels (via the mechanism of vascular endothelial growth factor or nitric oxide), thus inducing venous leakage (Mancini et al., 2005). Testosterone administration can potentially restore the estrogen/androgen imbalance.
It seems that we are entering an era of reassessment of the value of testosterone treatment in erectile dysfunction. In fact, several recent clinical evaluations have depicted a rather favorable profile of androgen administration to ageing men (Jain et al., 2000) but more well-designed studies are needed to assess the role of testosterone in improvement of erectile function.
For more information on the effect of TTh on different organ systems and/or prevention even in older men, we can refer to the recent published literature in this concern [19-23]. For the question: how long should TTh continue? Data suggests that interruption could cause recurrence in symptoms and signs of hypogonadism. So researchers agree to continue as lifetime treatment such as with Thyroxine or Insulin [24].