Clinical Case Reports and Clinical Study
OPEN ACCESS | Volume 13 - Issue 1 - 2026
ISSN No: 2766-8614 | Journal DOI: 10.61148/2766-8614/JCCRCS
Ahmad Pour-Rashidi1,2, Kimia Kazemzadeh1*, Sama Jabbaripour1,3
1Network of Neurosurgery and Artificial Intelligence (NONAI), Universal Scientific Education and Research Network (USERN), Tehran, Iran.
2Department of Neurosurgery, Sina Hospital, Tehran University of Medical Sciences, Tehran, Iran.
3School of Medicine, Tehran University of Medical Sciences, Tehran, Iran.
*Corresponding author: Kimia Kazemzadeh, Department of Neurosurgery, Sina Hospital, Tehran University of Medical Sciences, Tehran, Iran.
Received: December 08, 2025 | Accepted: December 23, 2025 | Published: January 06, 2026
Citation: Ahmad Pour-Rashidi, Kimia Kazemzadeh, Sama Jabbaripour. (2026) “Unraveling Predictors of Long-Term Survival (≥2 Years) in Glioblastoma; A Single Center Study” Clinical Case Reports and Clinical Study, 13(1); DOI: 10.61148/2766-8614/JCCRCS/224.
Copyright: © 2026 Kimia Kazemzadeh. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background: Glioblastoma multiforme (GBM) is the most common and aggressive primary brain tumor, characterized by a poor prognosis despite advancements in treatment. Long-term survival (LTS), defined as surviving beyond two years post-diagnosis, remains a critical goal for patient management. This study aims to identify key predictors of LTS in GBM patients, focusing on clinical and genetic factors.
Methods: A retrospective analysis was conducted on 41 newly diagnosed GBM patients treated at Sina Hospital, Iran. Patient data were collected, including demographics, clinical characteristics, and treatment modalities. Molecular analyses were performed to assess MGMT promoter methylation, IDH1, and TERT mutations. Statistical analyses utilized SPSS software to evaluate predictors of LTS through univariate and multivariate logistic regression models.
Results: The study identified significant predictors of LTS among GBM patients. Notably, 94.7% of long-term survivors had unifocal tumors compared to 54.5% of short-term survivors (p < 0.001). Higher Karnofsky Performance Status (KPS) scores at diagnosis were also associated with improved survival; LTS patients had a median KPS score of 100 versus 85 in non-LTS patients (p = 0.037). The final multivariate Cox regression analysis confirmed unifocal tumor status and elevated KPS scores as independent predictors of prolonged survival.
Conclusion: This study underscores the importance of specific clinical characteristics, such as unifocal tumor location and higher KPS scores, in predicting long-term survival in GBM patients. Although MGMT methylation showed a trend toward better survival outcomes, it did not achieve statistical significance in the logistic regression model. These findings provide valuable insights for enhancing prognostic assessments and developing targeted treatment strategies for improving patient outcomes.
Glioblastoma, Long-term survival, MGMT methylation, Karnofsky Performance Status, Unifocal tumors
Glioblastoma Multiforme (GBM) stayed uncured for a long time; thus, recent studies and treatments are trying to improve the patient’s quality of life and increase the survival rate (1). GBM is the most common intensive primary brain tumor with 60% properties between all brain gliomas (2) with a prevalence of 5-10 in every 100,000 people with an incidence of 14,000 patients just in the United States of America annually (3). GBM is the most dangerous and deadliest malignant brain tumor. The median survival with the best treatment combination such as maximal safe resection and chemo-radiotherapy progresses from 5 months (4) to 15 months (5) within these years. This means that the GBM prognosis is very poor even by new therapy techniques (6). The primary goal of glioblastoma multiforme (GBM) diagnosis and treatment has been to achieve long-term survival (LTS) for patients, defined as surviving two years or more following adequate extent of resection (EOR) and adjuvant therapies (1, 7).
Some factors are important for understanding LTS in patients. One critical factor is EOR, which refers to how much of a tumor is removed during surgery. Research shows that EOR has a significant impact on patient outcomes. In fact, evidence indicates that about 78% of patients experience notable effects related to EOR. The best long-term survival rates are observed when the extent of resection is 95% or greater (3). Based on the latest WHO classification for glioblastomas, 90% of which are branched into wildtype Isocitrate Dehydrogenase (IDH) and the other 10% are mutants 1 and 2 (5, 8), so IDH profile information may be helpful for LTS. Besides, specific genetic profiles of primary GBM included; amplification of epidermal growth factor receptor (EGFR), the telomerase reverse transcriptase (TERT) promoter, Methylation of the O-6-methylguanine-DNA methyltransferase (MGMT) addition to IDH 1 and 2 demonstrated as the main onco-markers for GBM (5, 7, 9-11). BRAF is a well-known proto-oncogene in human primary brain tumors. Over half of epithelioid GBMs have been reported to carry the BRAF V600E mutation. This uncommon clinical variant is more prevalent in young adults and has an equal representation of males and females in one series. BRAF has a special key effect on the growth signal transduction by encoding “B-Raf” serine/threonine-protein kinase that regulates the mitogen-activated protein kinase (MAPK) and extracellular signal-regulated kinase (ERK) signaling pathway (12, 13). Epithelioid glioblastoma (eGBM) as a GBM’s subgroup is the most favorite for BRAF V600E mutations with more than 50% properties and almost all of the BRAF mutations in GBM have a poor prognosis (14). MGMT promotor methylation showed significant relevance with better LTS (15, 16). Also, there are pieces of evidence about the association of Karnofsky performance status (KPS), age, and sex with LTS that proved younger patients (mostly below 50 years old) and higher KPS scores have better LTS (2, 6, 15-19). The more aggressive and complete treatment combination of maximal EOR and chemo-radiotherapy the better outcome for LTS (2, 6). We reported and designed this study in 41 GBM-confirmed patients to investigate the main predictors and their roles in LTS beyond 2 years.
2.1) Patient recruitment and data gathering
We enrolled 41 newly diagnosed GBM patients and retrospectively identified them by one of the first major neurosurgery centers in Iran; Sina hospital, data center. The study was approved by the local ethics committees at the contributing clinical centers. Histological diagnosis of GBM based on the World Health Organization (WHO) classification of brain tumors was confirmed by two pathologists. As it was mentioned, all the patients were the first line diagnosed with GBM, and recurrent or secondary cases were excluded. After selecting our population, we evaluated their conventional MRIs before the surgery. Moreover, for cases with eloquent cortex areas involvement such as motor cortex, Broca, or Wernicke’s area, we evaluated additional functional MRI (fMRI) and diffusion tensor imaging (DTI).
In this retrospective study, all the patients followed up for 24 months, and from the first month after surgery, every 3 months KPS score plus significant signs and symptoms were recorded in this study. Adjuvant therapies have been done for every single patient.
2.2) DNA Extraction
All 41 patients were involved in molecular analysis and gene recognition (They were based on the Sina Hospital’s protocol and standard of care for GBM patients). Formalin-fixed GBM tumor samples were encompassed with paraffin selected for DNA extracting according to the Reinfenberger et al. study in 1996 (20). For every one of the specimens used for nucleic acid extraction, more than 85% of the population was histologically assured of a GBM tumor cell content. Genetic profile information included MGMT promotor methylation, IDH-1, and TERT mutation registered in the database.
2.3) MGMT promotor methylation
Methylation of the O-6-methylguanine-DNA methyltransferase (MGMT) promoter has become one of the main prognostics and therapeutic GBM tumor markers and recent studies have shown the importance of MGMT in the treatment of glioblastoma. MGMT altered the DNA by deletion of alkyl groups in the guanine O6 gene area as a repair protein (7, 9). Also, MGMT promoter methylation is compatible with longer survival in patients treated with adjuvant therapy and temozolomide (TMZ) (21) as we had done in our study. MGMT promoter methylation was analyzed and recorded by methylation-specific polymerase chain reaction (PCR) according to the reported data by Mollemann et al. in 2005 (22). The primer sequences used to detect methylated MGMT promoter sequences were “5-GTT TTT AGA ACG TTT TGC GTT TCG AC-3 and 5-CAC CGT CCC GAA AAA AAA CTC CG-3”. The primer sequences used to detect unmethylated MGMT promoter sequences were “5-TGT GTT TTT AGA ATG TTT TGT GTT TTG AT-3 and 5-CTA CCA CCA TCC CAA AAA AAA ACT CCA-3”. We considered the A172 glioma cell line; which has an entirely methylated MGMT promoter; as a positive control sample. Also, an unmethylated control sample was considered from the intact brain tissue.
2.4) IDH-1 mutations
IDH mutations association with GBM tumor survival, especially the isolated ones, remained unpredictable as it inspired both long survival and mortality as well (9, 23). Each specimen was zinc-formalin-fixed and paraffin-embedded with hematoxylin-eosin-saffron (HES) and Masson trichrome. The whole immunohistochemical analyses were performed on this specific specimen. IDH1 specific part of exon 4, comprising the R132 mutation hotspot; however, the whole parts of exon 4 for IDH2 were amplified from genomic DNA by polymerase chain reaction (PCR), and the high-resolution melting curve analysis (HRM) was followed by sequence analysis (24). There are previous worthwhile studies that reported amplifications of a 122 bp base pairs length fragment spanning IDH1 (25) and a fragment of 290 bp base pairs length fragment spanning IDH2 entire exon 4 (26). Based on the HRM guidance on a Light Cycler 480 HRM analysis was performed and the result entered in our study database.
2.5) TERT mutation
Novel studies have been shown the importance of Telomerase reverse transcriptase (TERT) promoter mutations in progressing primary glioblastomas. Real-time quantitative PCR (qPCR) by Light Cycler 480 format recognized the mRNA expression levels of TERT and it was reported before by Arita et al (27). Moreover, Light Cycler 480 was using in Relative quantification analyses. TERT-specific primers, which are located in exon 5, were used from formalin-fixed paraffin-embedded samples:” GCCTGAGCTGTACTTTGTC” (P0155), and the reverse primer on exon 6: “CGTGTTCTGGGGTTTGATG” (P0156). TERT mRNA expression measurement was incompatible with human total brain RNA.
2.6) Sanger sequencing
To prepare the templates for Sanger sequencing, genomic DNA was amplified with the same primer pair as for Pyrosequencing without biotinylating the reverse primer using the BigDye Terminator Cycle Sequencing Kit v3.1.
2.7) Statistical analyses
All statistical analyses were performed using SPSS version 24.0 software (IBM, Armonk, New York), with a significance threshold set at P < 0.05. Descriptive statistics for continuous variables were expressed as mean ± standard deviation, while nominal variables were reported as counts and percentages. Continuous variable means were compared using independent samples t-tests, and nominal variable proportions were analyzed using the Chi-square test or Fisher’s exact test as appropriate. The study population was categorized into long-term survivors (LTS) and short-term survivors (non-LTS) to evaluate differences between these groups.
Univariate logistic regression was employed to identify predictors of LTS, with variables showing an odds ratio greater than one indicating an increased probability of long-term survival. Variables with p-values less than 0.25 from the univariate analysis were included in the multivariate logistic regression model, which utilized a forward stepwise approach based on the Wald statistic. Additionally, univariate Cox proportional hazards regression was conducted to identify independent predictors associated with overall survival, treating death as the event of interest. Kaplan-Meier survival plots were generated for categorical variables that demonstrated significant hazard ratios. The final multivariate Cox model included significant predictors: unifocal tumor location, initial KPS scores, and MGMT methylation percentage. Model performance metrics included Harrell's C-index of 0.8009 and Somers' D statistic of 0.6019, indicating strong predictive accuracy and a positive association between predicted outcomes and actual survival data.
In our analysis, we made several methodological adjustments to enhance the accuracy of our findings regarding predictors of LTS in GBM patients. To incorporate the methylation percentage as a variable, we defined a threshold where a methylation percentage of under 10% for MGMT-negative patients was considered as 0. Additionally, we simplified the tumor location variable, which originally included five categories (frontal, parietal, temporal, occipital, and multifocal), into two broader categories: unifocal and multifocal. This change aimed to improve the robustness of our results. We also focused on KPS scores as a critical outcome measure; since a KPS score of zero indicates death, we included follow-up KPS scores only at 1, 3, and 6 months in our regression analyses to reflect patient outcomes accurately, given that no deaths occurred within the first six months. Notably, all non-long-term survivors had a minimum survival of six months but succumbed prior to 21 months, a trend that was visually represented in the survival plots for both groups (check out Figure 1.).
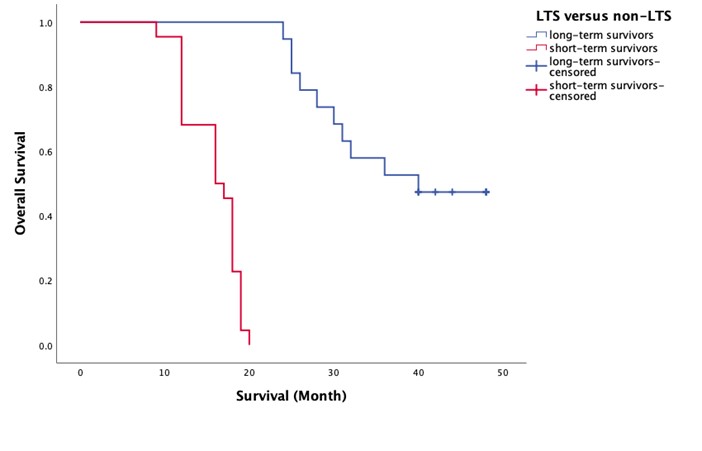
Figure 1. Survival plot of two groups
3.1) Clinical and biological characteristics of LTS and non-LTS of glioblastoma
The analysis of clinical and biological characteristics of glioblastoma patients revealed significant predictors of LTS versus non-LTS. Key findings indicated that unifocal tumors were strongly associated with LTS, with a notable percentage of LTS patients presenting with this characteristic (94.7%) compared to only 54.5% of non-LTS patients (p < 0.001). Additionally, the absence of a decline in the GCS at presentation was significantly correlated with LTS, as none of the LTS patients experienced a decline, while 22.7% of non-LTS patients did (p = 0.035). Higher KPS scores at diagnosis also emerged as a critical factor, with LTS patients having a median KPS score of 100 compared to 85 in non-LTS patients (p = 0.037). The multivariate logistic regression analysis further confirmed that unifocal tumor status and higher KPS scores at diagnosis were independent predictors of LTS, achieving an overall accuracy of 90.2% and an area under the curve (AUC) of 0.914, indicating robust model performance.
In terms of overall survival, univariate Cox proportional hazards regression highlighted that unifocal tumors, no GCS decline at presentation, higher initial KPS scores, and MGMT promoter methylation significantly contributed to longer survival times. The multivariate Cox regression analysis reinforced these findings, identifying unifocal tumor status and elevated KPS scores as independent predictors of prolonged survival. Notably, while MGMT methylation percentage did not show significant odds ratios in the logistic model, it was associated with better survival outcomes in the Cox model. The final model demonstrated strong predictive accuracy with a Harrell's C-index of 0.8009 and Somers' D statistic of 0.6019, underscoring the positive association between predicted outcomes and actual survival data. These results emphasize the importance of specific clinical characteristics in predicting both long-term survival and overall survival in glioblastoma patients, providing valuable insights for future prognostic assessments and treatment strategies.
Check out Table 1.
Table 2. LTS versus non-LTS predictors using univariate logistic regression by including constant in model (if >1 odds ratio, the variable indicates more probability of LTS)
|
|
short-term survivors (N=22) |
Long-term survivors (N=19) |
p-value |
|
Sex (%) |
|
|
|
|
Male |
15 (68.2%) |
11 (57.9%) |
0.495 |
|
Female |
7 (31.8%) |
8 (42.1%) |
|
|
Age at diagnosis (years) |
49.91± 10.85 |
44.74± 12.59 |
0.17 |
|
Tumor location |
|
|
|
|
Unifocal |
12 (54.5%) |
18 (94.7%) |
0.004* |
|
Frontal |
5 (41.67%) |
9 (50%) |
|
|
Parietal |
1 (8.33%) |
2 (11.11%) |
|
|
Temporal |
4 (33.33%) |
3 (16.67%) |
|
|
Occipital |
2 (16.67%) |
4 (22.22%) |
|
|
Multifocal |
10 (45.5%) |
1 (5.3%) |
|
|
Laterality |
|
|
|
|
Right hemisphere |
8 |
8 |
0.707 |
|
Left hemisphere |
14 |
11 |
|
|
Initial symptoms |
|
|
|
|
Declined GCS |
5 (22.7%) |
0 |
0.035* |
|
Muscular weakness |
5 (22.7%) |
4 (21.1%) |
1.00 |
|
hemiparesis |
1 (4.5%) |
4 (21.1%) |
0.164 |
|
hemiplegia |
4 (18.2%) |
0 |
0.111 |
|
Speech impairment |
2 (9.1%) |
2 (10.5%) |
1.00 |
|
Visual impairment |
2 (9.1%) |
2 (10.5%) |
1.00 |
|
Seizure |
5 (22.7%) |
10 (52.6%) |
0.047* |
|
Headache |
10 (45.5%) |
9 (47.4%) |
0.902 |
|
KPS at diagnosis |
85 (80–100) |
100 (90–100) |
0.037* |
|
KPS at follow-up of alive patients (months, IQR) |
|
|
|
|
1-month FU |
92.5 (78.75–100) |
90 (80–100) |
0.778 |
|
3-month FU |
95 (85–100) |
95 (90–100) |
0.705 |
|
6-month FU |
90 (80–100) |
95 (90–100) |
0.203 |
|
9-month FU |
90 (80–97.5) |
95 (90–100) |
0.085 |
|
12-month FU |
90 (80–100) |
95 (90–100) |
0.085 |
|
15-month FU |
80 (70–90) |
95 (90–100) |
<0.001* |
|
18-month FU |
80 (80–80) |
95 (90–100) |
0.003* |
|
21-month FU |
|
95 (80–100) |
|
|
24-month FU |
|
95 (80–100) |
|
|
Genetic mutations |
|
|
|
|
Mutant IDH1 (%) |
4 (18.2%) |
4 (21.1%) |
1.00 |
|
Mutant TERT (%) |
6 (27.3%) |
5 (26.3%) |
0.945 |
|
Methylated MGMT (%) |
9 (40.9%) |
13 (68.4%) |
0.078 |
|
MGMT methylation percentage |
0 (0 – 16.25) |
20 (0 – 25) |
0.054 |
|
Therapeutic approach |
|
|
|
|
Routine |
19 |
16 |
1.00 |
|
Awake |
3 |
3 |
|
|
Extent of resection |
97.5 (85-100) |
100 (95-100) |
0.309 |
|
overall survival (months) |
15.82± 3.22 |
36.58± 8.69 |
<0.001* |
|
6-month survival rate |
22 (100%) |
19 (100%) |
|
|
12-month survival rate |
21 (95.5%) |
19 (100%) |
1.00 |
|
18-month survival rate |
10 (45.5%) |
19 (100%) |
0.00013* |
* Statistically significant
Table 1. Summarization of the clinical and biological characteristics of short-term survivors versus long-term survivors
3.2) LTS versus Non-LTS Predictors Using Logistic Regression
3.2.1) Univariate logistic regression
The univariate logistic regression analysis identified several predictors associated with LTS in glioblastoma patients. Among the variables evaluated, the presence of a unifocal tumor was significantly associated with LTS, yielding an odds ratio (OR) of 15.00 (95% CI: 1.693 – 132.90, p = 0.015), indicating that patients with tumors confined to one lobe were substantially more likely to achieve long-term survival compared to those with multifocal tumors. Additionally, a higher initial KPS score at diagnosis was found to be a significant predictor of LTS, with an OR of 1.067 (95% CI: 1.001 – 1.138, p = 0.046). The analysis also suggested that the presence of seizures at presentation approached significance with an OR of 3.778 (95% CI: 0.986 – 14.479, p = 0.053), indicating a potential positive correlation with long-term outcomes.
In contrast, other variables such as age, sex, and tumor laterality did not demonstrate significant associations with LTS, as evidenced by their respective p-values exceeding the threshold of 0.05. Notably, the decline in GCS at presentation was not applicable for LTS patients, suggesting that a stable neurological status may be crucial for achieving better survival outcomes. The multivariate logistic regression analysis further reinforced the findings from the univariate model, confirming that unifocal tumor status and higher initial KPS scores remained independent predictors of LTS after adjusting for other factors. The final model exhibited strong performance metrics, including a Cox and Snell R² value of 0.447, indicating that approximately 44.7% of the variance in LTS could be explained by the model, along with an overall accuracy of 90.2% and an AUC of 0.914, demonstrating excellent classification capability between LTS and non-LTS patients.
Check out Table 2.
Table 2. LTS versus non-LTS predictors using univariate logistic regression by including constant in model (if >1 odds ratio, the variable indicates more probability of LTS).
|
variable |
p-value |
Odds ratio |
95%CI |
|
Male |
0.496 |
0.642 |
0.179 – 2.304 |
|
Age |
0.164 |
0.962 |
0.910 – 1.016 |
|
Unifocal (being in only one lobe) |
0.015* |
15.00 |
1.693 – 132.90 |
|
Right hemisphere location |
0.707 |
1.273 |
0.362 – 4.480 |
|
Declined GCS |
0.999 |
0.000 |
0.000 - . |
|
Muscular weakness |
0.897 |
0.907 |
0.205 – 4.010 |
|
Speech impairment |
0.877 |
1.176 |
0.149 – 9.266 |
|
Visual impairment |
0.877 |
1.176 |
0.149 – 9.266 |
|
Seizure |
0.053 |
3.778 |
0.986 – 14.479 |
|
Headache |
0.902 |
1.080 |
0.315 – 3.698 |
|
Initial KPS |
0.046* |
1.067 |
1.001 – 1.138 |
|
IDH1 mutation |
0.817 |
1.200 |
0.256 – 5.631 |
|
TERT mutation |
0.945 |
0.952 |
0.238 – 3.811 |
|
MGMT methylation |
0.082 |
3.130 |
0.864 – 11.343 |
|
MGMT methylation percentage |
0.087 |
1.048 |
0.993 – 1.106 |
|
Routine therapeutic approach |
0.846 |
0.842 |
0.149 – 4.764 |
|
Extend of resection |
0.179 |
1.070 |
0.969 – 1.182 |
|
KPS 1M |
0.901 |
1.003 |
0.956 – 1.052 |
|
KPS 3M |
0.845 |
1.006 |
0.950 – 1.064 |
|
KPS 6M |
0.314 |
1.035 |
0.968 – 1.107 |
* Statistically significant (p-value =<0.05)
3.2.2) Multivariate logistic regression
In the multivariate logistic regression analysis, we focused on variables that demonstrated significance in the univariate analysis (p < 0.25) and those previously associated with LTS. The selected variables included age, tumor location (unifocal), presence of seizures, initial KPS, MGMT methylation status, MGMT methylation percentage, extent of resection, as well as IDH1 and TERT mutations. A forward stepwise approach based on the Wald statistic was employed to identify the fittest model (See Table. 3). The results indicated that unifocal tumor location was a strong independent predictor of LTS, with a coefficient (B) of 3.96 and an adjusted OR of 52.36 (95% CI: 3.74 – 733.99, p = 0.003). Additionally, higher initial KPS scores were also significantly associated with LTS, yielding a coefficient of 0.12 and an adjusted OR of 1.13 (95% CI: 1.03 – 1.23, p = 0.011). Although MGMT methylation percentage did not achieve statistical significance with a p-value of 0.082, its inclusion in the model notably increased classification accuracy from 78% to 90%, highlighting its potential relevance in predicting long-term outcomes.
Table 3. Fittest model using a forward stepwise approach based on the Wald statistic
|
Variable |
B (coefficient) |
Adjusted OR (95% CI) |
P-valuea |
|
location (unifocal) |
3.96 |
52.36 (3.74 – 733.99) |
0.003 |
|
Initial KPS |
0.12 |
1.13 (1.03 – 1.23) |
0.011 |
|
MGMT methylation percentage |
0.07 |
1.07 (0.99 – 1.15) |
0.082 |
Wald test
The final model performance metrics underscored its robustness in predicting LTS among glioblastoma patients. The Cox and Snell R² value was calculated at 0.447, indicating that the model explained approximately 44.7% of the variance in LTS outcomes. The overall accuracy reached an impressive 90.2%, reflecting the proportion of correctly classified cases based on a cut-off value of 0.5. The classification table demonstrated that out of 22 observed LTS patients, 20 were correctly predicted as LTS (90.9%), while among the non-LTS group, 17 out of 19 were accurately classified (89.5%) (See Table 4.)
Table 4. Classification Table (with the cut value of 0.5)
|
Observed |
|
Predicted |
||
|
LTS |
Percentage Correct |
|||
|
0 |
1 |
|||
|
LTS |
0 |
20 |
2 |
90.9 |
|
1 |
2 |
17 |
89.5 |
|
|
Overall Percentage |
|
|
90.2 |
|
Furthermore, the AUC was determined to be 0.914, signifying excellent performance in distinguishing between LTS and non-LTS patients through receiver operating characteristic (ROC) analysis (Check out Figure 2.).
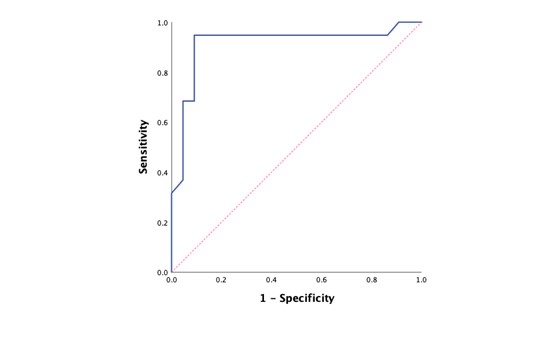
Figure 2. ROC curve for predicting long-term survival based on predicted probabilities of the model
3.3) Univariate cox proportional hazards regression
The univariate Cox proportional hazards regression analysis was conducted to identify independent predictors associated with overall survival in glioblastoma patients, treating death as the event of interest. Several variables were analyzed, revealing significant associations with survival outcomes. Notably, the presence of a unifocal tumor was identified as a strong predictor of longer survival, with a hazard ratio (HR) of 0.310 (95% CI: 0.134 – 0.716, p = 0.006). This indicates that patients with unifocal tumors had a significantly lower risk of death compared to those with multifocal tumors. Additionally, the analysis showed that a decline in the GCS at presentation was associated with poorer survival outcomes, yielding an HR of 4.834 (95% CI: 1.731 – 13.501, p = 0.003). Higher initial KPS scores also correlated positively with longer survival, with an HR of 0.963 (95% CI: 0.941 – 0.987, p = 0.002), suggesting that better functional status at diagnosis is linked to improved survival rates.
Further analysis revealed that MGMT promoter methylation was significantly associated with longer survival (HR = 0.449, 95% CI: 0.220 – 0.916, p = 0.028), as well as the percentage of MGMT methylation (HR = 0.965, 95% CI: 0.934 – 0.996, p = 0.027). These findings indicate that both the presence and extent of MGMT methylation contribute positively to survival outcomes in glioblastoma patients. Other variables such as age, sex, and tumor laterality did not demonstrate significant associations with overall survival, as their p-values exceeded the threshold of significance (p > 0.05).
Check out Table 5.
Table 5. Univariate cox proportional hazards regression to identify independent predictors associated with overall survival instead of being LTS versus non-LTS
|
variable |
p-value |
Hazards ratio |
95%CI |
|
Male |
0.741 |
1.129 |
0.551 – 2.313 |
|
Age |
0.138 |
1.022 |
0.993 – 1.053 |
|
Unifocal tumor |
0.006* |
0.310 |
0.134 – 0.716 |
|
Right hemisphere location |
0.570 |
0.809 |
0.390 – 1.680 |
|
Declined GCS |
0.003* |
4.834 |
1.731 – 13.501 |
|
Muscular weakness |
0.485 |
1.331 |
0.597 – 2.971 |
|
Speech impairment |
0.634 |
0.749 |
0.228 – 2.462 |
|
Visual impairment |
0.880 |
0.912 |
0.277 – 3.000 |
|
Seizure |
0.204 |
0.611 |
0.285 – 1.307 |
|
Headache |
0.567 |
1.229 |
0.607 – 2.488 |
|
Initial KPS |
0.002* |
0.963 |
0.941 – 0.987 |
|
IDH1 mutation |
0.473 |
0.722 |
0.296 – 1.759 |
|
TERT mutation |
0.352 |
1.445 |
0.665 – 3.139 |
|
MGMT methylation |
0.028* |
0.449 |
0.220 – 0.916 |
|
MGMT methylation percentage |
0.027* |
0.965 |
0.934 – 0.996 |
|
Routine therapeutic approach |
0.954 |
0.973 |
0.374 – 2.529 |
|
Extend of resection |
0.127 |
0.961 |
0.914 – 1.011 |
|
KPS 1M |
0.830 |
0.997 |
0.972 – 1.023 |
|
KPS 3M |
0.889 |
1.002 |
0.973 – 1.032 |
|
KPS 6M |
0.609 |
0.991 |
0.960 – 1.024 |
* Statistically significant
- Death is considered the event.
3.4) Kaplan Meier survival plots
The Kaplan-Meier survival analysis was performed to visualize overall survival (OS) based on categorical variables that demonstrated significant hazard ratios in the univariate Cox proportional hazards regression analysis. The results indicated that MGMT methylation status, GCS decline at presentation, and tumor location significantly influenced survival outcomes among glioblastoma patients.
The mean overall survival for patients with non-methylated MGMT status was 20.74 months (median: 18 months), compared to 30.68 months (median: 28 months) for those with methylated MGMT status. The log-rank test yielded a Chi-square value of 5.550 with a p-value of 0.018, indicating a statistically significant difference in survival between the two groups (See Figure 3.).
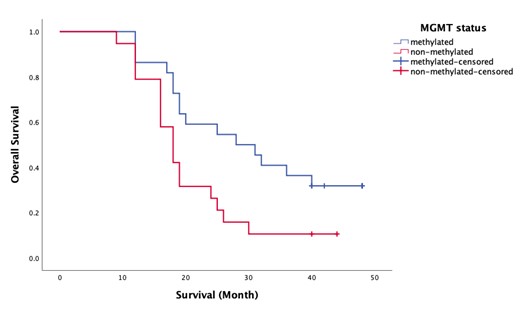
Figure 3. Kaplan Meier survival plot for MGMT status
The analysis also revealed a significant impact of GCS decline at presentation on overall survival. Patients who did not experience a decline in GCS had a mean OS of 28.03 months (median: 24 months), while those with a decline had a mean OS of only 13.60 months (median: 12 months). The log-rank test produced a Chi-square value of 12.33 and a p-value of 0.000, demonstrating a highly significant difference in survival outcomes based on GCS status. This result emphasizes that maintaining neurological function at diagnosis is critical for improving survival prospects.
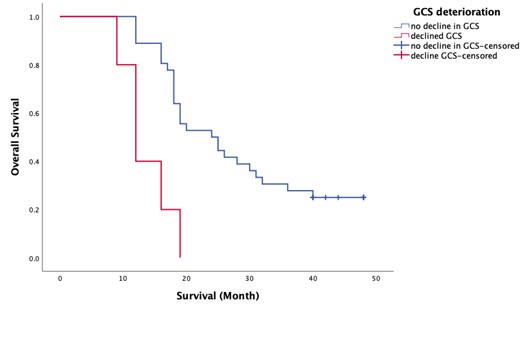
Figure 4. Kaplan Meier survival plot for GCS deterioration
Lastly, tumor location was also found to significantly affect overall survival. Patients with unifocal tumors had a mean OS of 29.63 months (median: 26 months), while those with multifocal tumors had a mean OS of 17.09 months (median: 18 months). The log-rank test indicated a Chi-square value of 9.42 with a p-value of 0.002, confirming that unifocal tumor status is associated with better survival outcomes compared to multifocal tumors (Check out Figure 5.).
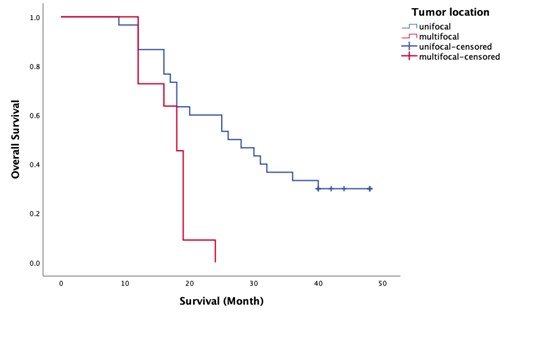
Figure 5. Kaplan Meier survival plot for tumor location
See Table 6.
Table 6. Kaplan Meier survival plots only for the categorical variables shown to have significant hazards ratio
|
Variable |
Mean (median) OS for 0 |
Mean (median) OS for 1 |
log rank test |
|
|
Chi-square |
P-value |
|||
|
MGMT status (0=non-methylated) |
20.74 (18) |
30.68 (28) |
5.550 |
0.018 |
|
Decline in GCS (0=no decline) |
28.03 (24) |
13.60 (12) |
12.33 |
0.000 |
|
Tumor location (0=unifocal) |
29.63 (26) |
17.09 (18) |
9.42 |
0.002 |
3.5) Multivariate cox proportional hazards regression
In the multivariate Cox proportional hazards regression analysis, we aimed to identify independent predictors associated with overall survival in glioblastoma patients. The variables selected for this analysis included those with a p-value of less than 0.25 from the univariate analysis, which comprised tumor location (unifocal vs. multifocal), deterioration in GCS at presentation, initial KPS, MGMT methylation status, MGMT methylation percentage, age, presence of seizures, and extent of resection. Additionally, IDH1 and TERT mutations were included due to their previously established association with long-term survival. A forward stepwise approach based on the Wald statistic was utilized to determine the fittest model.
The fittest model revealed three significant independent predictors of longer survival: unifocal tumor location, higher initial KPS scores, and MGMT promoter methylation percentage. Specifically, unifocal tumors were associated with a HR of 0.22 (95% CI: 0.09 – 0.56, p = 0.001), indicating a significantly reduced risk of death compared to multifocal tumors. The initial KPS score also emerged as a critical factor, with a coefficient of -0.069 and an HR of 0.93 (95% CI: 0.91 – 0.96, p = 0.000), suggesting that better functional status at diagnosis correlates with improved survival outcomes. Furthermore, the MGMT methylation percentage had a coefficient of -0.042 and an HR of 0.96 (95% CI: 0.93 – 0.99, p = 0.013), indicating that higher levels of MGMT methylation are associated with a lower risk of mortality (See Table 7.).
Table 7. Fittest model (using a forward stepwise approach based on the Wald statistic)
|
Variable |
B (coefficient) |
Adjusted HR (95% CI) |
P-valuea |
|
location (unifocal) |
-1.508 |
0.22 (0.09 – 0.56) |
0.001 |
|
Initial KPS |
-0.069 |
0.93 (0.91 – 0.96) |
0.000 |
|
MGMT methylation percentage |
-0.042 |
0.96 (0.93 – 0.99) |
0.013 |
a.Wald test
The performance metrics of the final model demonstrated strong predictive accuracy, with Harrell's C-index calculated at 0.8009, indicating that approximately 80% of the time, the model accurately predicts which patient will experience an event first. Additionally, Somers' D statistic was recorded at 0.6019, reflecting a strong positive association between the model's predictions and actual survival outcomes.
In our study, we aimed to identify predictors of LTS in patients diagnosed with GBM at a single center. Our analysis included 41 patients, and we found that unifocal tumor status and higher KPS scores at diagnosis were significant predictors of LTS, with an overall accuracy of 90.2% in our multivariate logistic regression model. Additionally, we observed that MGMT promoter methylation was associated with better survival outcomes, although it did not show significant odds ratios in the logistic model.
Our findings align with some previous studies that have highlighted the importance of tumor characteristics and patient performance status in predicting outcomes for GBM patients. The EORTC 1419 ETERNITY study focused on long-term survivors (≥5 years) of glioblastoma. It identified that freedom from progression (recurrence-free status) is a strong predictor of overall survival. Among the studied cohort, the median overall survival was notably high at 9.9 years. The study highlighted that patients without tumor recurrence had significantly longer survival compared to those with recurrences, emphasizing the role of tumor genetics such as MGMT promoter methylation in treatment response (28, 29). Research by Aaron Cohen-Gadol emphasized that younger age at diagnosis and favorable tumor genetics (specifically MGMT promoter methylation and IDH mutations) are critical predictors of long-term survival. Patients with MGMT methylation had a median survival time extending up to 22 months, while those with IDH mutations showed even longer median survival times around 31 months. This underscores the importance of genetic profiling in predicting outcomes (30).
Another significant finding came from a study examining IDH wildtype glioblastoma patients, which reported a median overall survival of 9.9 years among those who were free from recurrence. Notably, a substantial portion (approximately 74%) had tumors with MGMT promoter methylation, indicating that this genetic factor plays a pivotal role in enhancing treatment efficacy and overall survival (28, 29). Another study examined the long-term survival of six GBM patients who survived for over three years, a rarity given that only 3-6% of GBM patients achieve such longevity. The mean age of these patients was 25.7 years, and all underwent postoperative radiotherapy with an average dose of 55 gray. Four patients received nitrosourea-based chemotherapy. The results indicated a mean survival of 5.2 years, emphasizing that younger age and complete surgical resection are critical factors associated with long-term survival in GBM patients (31).
A comprehensive study analyzed the clinical and molecular characteristics of 23 LTS of glioblastoma compared to short-term survivors (STS). The study found that LTS were generally younger and had tumors enriched for MGMT promoter methylation and TP53 mutations. Notably, diagnostic MRIs showed more LTS with T1 tumor hypointensity, indicating distinct imaging features associated with better prognosis. This study underscored the importance of integrating molecular diagnostics into clinical evaluations to accurately predict individual patient outcomes (32).
The findings from another study indicate that glioma patients with IDH1 mutations have a median OS of 21 months, compared to 17.5 months for those without such mutations. Additionally, patients with methylated MGMT promoters exhibited a median OS of 21 months, while those with unmethylated MGMT had a median OS of 19 months. The combination of IDH1 mutation and MGMT methylation provides a more precise prediction of survival in glioblastoma than either marker alone. Patients were classified into three distinct genotypes based on their IDH1 and MGMT status: those with mutant IDH1 and methylated MGMT had the best survival rates, followed by those with either mutant IDH1 and unmethylated MGMT or wildtype IDH1 and methylated MGMT, while those with wildtype IDH1 and unmethylated MGMT had the shortest survival. These molecular characteristics, particularly MGMT methylation and IDH1 mutations, have been associated with improved prognosis in GBM, as they enhance the effectiveness of temozolomide therapy. Furthermore, co-methylation of IDH and MGMT is linked to better prognostic outcomes and can predict responses to chemotherapy and surgical interventions (33, 34).
A study of adult patients with non-H3-altered grade 4 gliomas who underwent maximal safe resection and adjuvant therapy from January 2019 to January 2021 revealed that the average OS was 14.45 months, and progression-free survival (PFS) was 10.66 months. Patients with TERTp mutations experienced significantly shorter OS (10.9 months) and PFS (6.9 months) compared to those without these mutations (15.9 months OS and 12.3 months PFS). Additionally, IDH mutation and TERTp wildtype status were associated with better survival outcomes. Notably, preoperative KPS scores were more predictive of patient outcomes than genetic factors, while MGMT and EGFR statuses did not provide significant prognostic insights in this study (35).
Despite the significant findings, our study has several limitations that must be acknowledged. First, the sample size of 41 patients is relatively small, which may limit the generalizability of our results. A larger cohort would provide more robust statistical power and potentially reveal additional predictors of LTS. Second, this study was conducted at a single center, which may introduce selection bias. The patient population may not be representative of the broader GBM population due to variations in treatment protocols and demographic factors across different centers or regions. Third, our retrospective design inherently limits the ability to establish causality between identified predictors and long-term survival. While we employed rigorous statistical analyses to control for confounding variables, prospective studies are needed to validate our findings.
In this retrospective study, we noted that a significant percentage of long-term survivors exhibited high levels of MGMT promoter methylation, which is associated with better survival outcomes. The majority of long-term survivors had unifocal tumors (94.7%), indicating that tumor location plays a crucial role in survival prospects. Also, Higher initial KPS scores were observed in long-term survivors, emphasizing the importance of overall patient health at diagnosis. The insights gained from our study underscore the need for further investigation into the molecular and genetic underpinnings of GBM that contribute to long-term survival. For instance, while MGMT methylation has been extensively studied as a prognostic marker, its role could be explored further in conjunction with other genetic mutations such as TERT or BRAF mutations. Moreover, exploring the impact of treatment modalities beyond standard care—such as immunotherapy or novel targeted therapies—on LTS could yield valuable information that enhances patient management strategies.
Overall, our study provides important insights into predictors of long-term survival in GBM patients while highlighting key limitations that warrant caution in interpreting the results. By addressing these limitations and expanding future research efforts, we can improve our understanding of GBM prognosis and ultimately enhance patient outcomes.
Declarations:
Funding: None.
Acknowledgments: None to declare.
Data availability statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflict of interest: The authors had no competing interests.
Ethics approval statement: The study protocol was approved by the institutional research ethics committee in Sina hospital (IR.TUMS.SINAHOSPITAL.REC.1402.121).
Patient consent statement: Informed consent was obtained from all patients.