Clinical Case Reports and Clinical Study
OPEN ACCESS | Volume 13 - Issue 1 - 2026
ISSN No: 2766-8614 | Journal DOI: 10.61148/2766-8614/JCCRCS
Mahalakshmi V, Thorat D, Bhattacharyya S*, Banik A, Raj A
Dept. of Microbiology, All India Institute of Hygiene and Public Health Kolkata, West Bengal India
*Corresponding author: Bhattacharyya S, Dept. of Microbiology, All India Institute of Hygiene and Public Health Kolkata, West Bengal India
Received: June 23, 2021
Accepted: June 28, 2021
Published: July 07, 2021
Citation: Mahalakshmi V, Thorat D, Bhattacharyya S, Banik A, Raj A “Prevalence of Brucellosis in Dairy Cattle in Singur – Hooghly, West Bengal Using on Spot Testing Techniques”. Clinical Case Reports and Clinical Study, 4(5); DOI: 10.61148/2766-8614/JCCRCS/083
Copyright: © 2021 Bhattacharyya S. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Brucellosis is one of the important common zoonotic diseases that occurs globally especially in both developed and developing countries. Although in some countries control and eradication measures were taken but still happens to exist in huge numbers because of its wide host range. Human transmission is mostly from animal reservoirs and daily cattle is the common source. The study region Singur in Hooghly, West Bengal is one of the major contributors of milk among the state. Samples were collected from the lactating cows of the region and Milk Ring test was conducted using Abortus Bang Ring Test colored antigen. In this study out of 62 animals testes 16.92% were positive for Brucellosis Milk ring test. Early diagnosis is necessary in prevention of any disease. Milk Ring test is an easy, convenient spot test for early diagnosis of Bovine Brucellosis among the dairy cattle population. This study aims in detecting the prevalence of bovine brucellosis among the study region.
Introduction:
Brucellosis causes huge economic loss among the farmers in both developed and developing countries. The disease is widespread and causes significant public health problems (1). Apparently 5,00,000 cases are reported annually worldwide which is estimated to be the real number of Brucella affected individuals are 26% higher than the actual reported cases (2). It is an important zoonotic disease affecting humans and domestic animals like Cattle, sheep, goat, pigs and dogs (3). Brucella spp are not host specific and the only source of transmission to humans being the animal reservoir (4). It is caused mainly by Brucella abortus, B. melitensis and B. suis which are not host specific and cause zoonotic disease in humans (3). Regions like Mediterranean, Indian subcontinent and several Asian states are endemic and prevalent to Brucellosis (5).
The microbe causing Brucellosis is a gram negative intracellular, pleomorphic organism which primarily causes infectious abortion in cattle (Bang’s Disease) and swine. Abortions are most common during outbreaks and primarily occurs in unvaccinated heifers over 5 months pregnant (6). Brucellae are non motile, non spore forming and are highly infectious even in small numbers. Among 6 species in the genus Brucella, three of them cause important human diseases. They are Brucella melitensis of goats and sheep, biotypes 1–3; B. abortus of cattle, biotypes 1–9; and B. suis of pigs, European hares, and reindeer, biotypes 1–4, with the possible exception of biovar 2. The disease distributed worldwide plays an important role in occupational zoonosis for those who are in contact with livestock and animals like farmers, veterinarian, slaughterhouse workers, meat industry workers and laboratory personnel (7).
Animals are the only source of infection and transmission occurs through direct and indirect contact. Consumption of contaminated or unpasteurized dairy products, direct contact with infected animals tissues and also by aerosol route causes infection. Human to human transmission is rare (8). Following inoculation of the organism incubation ranges from 1 to 5 weeks and there are either possibilities for the infection to be symptomatic or asymptomatic. Symptoms in humans include fever, sweats, myalgia, back ache, anorexia, headache and arthralgia. Chronic form of brucellosis occurs commonly in geriatric patients manifested as Chronic fatigue syndrome and localized infection (9).
Brucellosis being a notable public health concern disease in developing countries, often coexists with Tuberculosis. So the treatments given for brucellosis have high chances of producing antibiotic resistance in Mycobacteria and vice versa (10). According to World Health Organization (WHO) mass immunization can be the only way to reduce the spread of Brucellosis. B. abortus strain 19 attenuated vaccine has been widely used. Although it solely cannot eradicate the disease it helps in reducing the cases of abortion and reducing the spread (11). Diagnosis commonly includes a number of serological tests, since the infected cattle does not necessarily produce all the antibodies in detectable levels. Serological tests like Plate Agglutination Test, Compliment Fixation Test (CFT), Enzyme Linked Immuno Sorbent Assay (ELISA), Fluorescence Polarization Assay (FPA) can be used to screen herd or flock.Milk Ring Test and ELISA performed in milk sample is an effective screening and monitoring method for dairy cattle at field level (12). In a highly prevalent area, a diagnosis test with adequate sensitivity and high specificity is recommended to minimize the false positive cases, whereas in low prevalence areas, a test with sufficient specificity but high sensitivity is desirable (13). Final determination of the status of the herd or individual animal is accomplished by blood testing. The more frequently a herd is tested for MRT, the more effective the test becomes as a method to detect early infections preventing serious outbreaks (7). Early diagnosis and prevention of disease in animals and livestock is a key feature to be considered in controlling Brucellosis worldwide. This study was aimed at detecting the prevalence of Brucellosis in the rural parts of West Bengal, Hooghly region.
Materials and Methods :
Study population : Cows of Singur block, Hooghly district, West Bengal
Study type: Observational study
Sample size: 65 cows
Sampling type : Purposive sampling
Duration of Study : December 2020 to January 2021
Study area : Hooghly district in West bengal is one among the major milk producing areas in the state with an average milk production of 300,000 MT according to NDDB (National Dairy Development Board) data of the year 2015-16 (14).
Three small villages of the Singur block were selected based on Purposive sampling which included 25 households with an average herd size ranging from 1 to 10 animals. 65 cows were sampled for both Brucella Milk Ring Test and CMT for detecting Subclinical mastitis. The animal owners were interviewed using preformed questionnaires to check the hygienic animal husbandry practices. The animals were examined generally and they were found to be apparently healthy. Artificial insemination is the common method of reproduction followed in the study area.
Figure 1: Hooghly district map showing chosen area for study
Collection of Milk Sample
Milk samples were collected according to Standard guidelines (22). The udder of the animal was washed and the first stream of milk was discarded. A total of 5-10 ml of milk sample was collected in a sterile container for Abortus Bang Ring Test from all the quarters. The milk sample was stored in a portable vaccine carrier with an ice pack to maintain cold chain before bringing it to the laboratory
California Mastitis Test (CMT):
California Mastitis Test (24) was performed with the use of readymade CMT kit SyrVet manufactured by Kissan Dairy Machinery, Bangalore. 1-2 ml of milk was collected in respective paddles keeping the quarters separate. Excess milk was drained away and an equal amount of reagent added and mixed by swirling movement for 30 seconds. The results were read based on the color intensity and gel formation as given by the manufacturer.
Abortus Bang Ring Test:
The Milk Ring Test was performed according to the guidelines of OIE Terrestrial Manual 2018 (23). The antigen used was procured from IVPM (Institute of Veterinary Preventive Medicine), Ranipet which is a cell suspension of Brucella abortus Strain S-99 with an indicator dye 235 triphenyl tetrazolium powder. 50 µL of antigen was added to 2 ml of milk sample and incubated at 37°C for 1 hour at the microbiology lab at RH&UTC(Rural Health Unit and Training centre) Singur. The positive test result was indicated by the formation of pink colored creamy layer at the top and negative results were indicated by the uniform pink color of the milk column.
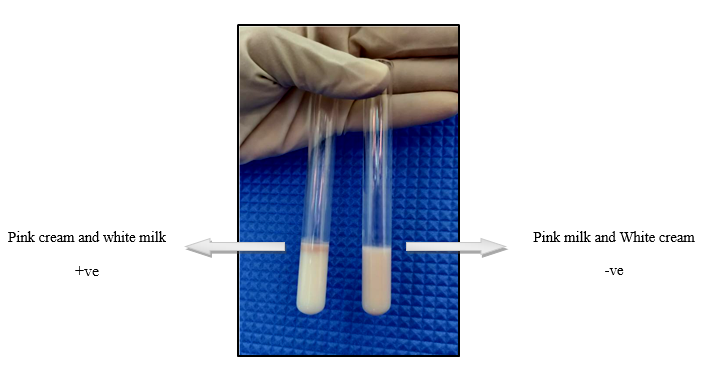
Figure 2: Milk Ring Test Results
Results:
The positive reaction was indicated by the pink colour cream at the top of the column. The animals which has developed antibodies (agglutinins) to Brucellosis which will usually be carried by the cream react with the antigen and form the pink coloured creamy layer at the top of the layer. Any presence of agglutinins in the milk will be carried by the cream and reacts with the antigen in its presence. In the absence of antibodies the antigen will be free which is detected as the pink coloured milk and white coloured cream at the top layer(22).
Among 65 animals tested 11 cases showed positive results for the Milk Ring Test which is as approximately 16.92 % of positive cases from the study population. So milk ring test can be taken as an easy preliminary screening test in field level which has a high sensitivity among the other diagnostic tests for Brucella (20). California Mastitis Test showed a total of 16 positive cases. Soe MRT negatie cases were also positive for CMT indicating the less specificity of the Milk Ring Test (15).
Discussion
The study shows that the area has a high prevalence of Brucellosis with the 16.92% positive result for the Milk Ring Test. The significant interaction of the subclinical mastitis and MRT positive cases is of major concern. Due to the limitations of MRT which lacks specificity of its own, other confirmatory serological tests like Compliment Fixation Test (CFT), Enzyme Linked Immuno Sorbent Assay (ELISA) or Polymerised Chain Reaction (PCR) should be carried out for the suspected cases (20). California mastitis test is reportedly about 80% sensitive, and hence can still be used.
Majority of the farmers in the study area were following mixed farming of rearing small ruminants, ducks and cattle together. This type of mixed rearing also is a risk factor to the epizootic occurrence of brucellosis in many parts of the world (16). Although various control and eradication programmes have been implemented all over the country the information regarding the prevalence of brucellosis in the animal population is still not sufficient.
Ingestion of contaminated milk and dairy products is one of the major sources of transmission in humans even in non endemic regions of the world (17). Early diagnosis plays a key role in control and eradication of the disease in the economically poor parts of the country. Sensitivity is a major factor in the early diagnosis for which milk antibodies are easily detected using MRT (18).
Control and prevention of animal brucellosis is a crucial step in control of human brucellosis (19). Even though MRT lacks its sensitivity, it is an easy and stable spot on test for field diagnosis of brucellosis in herd level. Periodical surveillance in herd level is required to achieve eradication of the disease in a public health point of view (21).
Acknowledgements:
The authors thank Rural Health Unit and Training Institute, Singur and the Public Health workers of the same, Department of Microbiology and Department of Epidemiology, All India Institute of Hygiene and Public Health, Kolkata for providing necessary help in conducting the study.