Clinical Case Reports and Clinical Study
OPEN ACCESS | Volume 13 - Issue 1 - 2026
ISSN No: 2766-8614 | Journal DOI: 10.61148/2766-8614/JCCRCS
Abdelrazak Mansour Ali 1, *, Mohamed Abdeltawab Ibrahim2, and Radwa Abdelrazak Ali 3.
1Professor Doctor, (MD, PhD). Corresponding Author, Professor of Pediatrics, International Center for population studies & research, Al-Azhar University, Cairo, Egypt.
2Doctor of Medicine (MD). Ministry of Health, General Director of Marsa Alam Hospital, Quality management consultant, Cairo, Egypt.
3Associate Researcher, George Mason University, Neuroscience specialty., National Institute of Health, Research Department. USA.
*Corresponding author: Abdelrazak Mansour Ali, Pediatrics, International Center for population studies & research, Al-Azhar University, Cairo, Egypt.
Received: January 04, 2025
Accepted: January 10, 2025
Published: January 15, 2025
Citation: Abdelrazak Mansour Ali, Mohamed Abdeltawab Ibrahim, and Radwa Abdelrazak Ali (2025). “Interrelation between Helicobacter Pylori, Methanogens, Food, and Functional Dyspeptic Syndromes.”. Clinical Case Reports and Clinical Study, 12(1); DOI: 10.61148/2766- 8614/JCCRCS/194
Copyright: © 2025. Abdelrazak Mansour Ali. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The microbe-host interaction in the intestinal tract underlies many human disorders, including disorders of gut-brain interactions, previously termed functional bowel disorders, such as irritable bowel syndrome (IBS) and small intestinal bacterial overgrowth (SIBO).
Objective: To determine whether Helicobacter pylori infection is associated with irritable bowel syndrome, small bowel bacterial overgrowth, and other functional gut disorders.
Design: Case-control retrospective study at local tertiary hospitals in Egypt.
Methods: A total of 125 cases of IBS that met Rome IV criteria, 100 cases with SIBO, and 100 controls (IBS and SIBO-negative), aged 18 to 60 years old, were involved in this study. Patients were recruited from August 2023 to July 2024, and matched by age, sex, and other demographic variables. All participants were tested for urea breath test, lactulose hydrogen‐methane breath test, and H pylori stool antigen tests.
Results. Of the total 225 cases, 125 (55.6%) had reported IBS, a 100 (44.4%) had SIBO, compared to 100 in the control group. H. pylori was present in 109 (48.4%) of cases compared to only 16 (16.0%) in the control group. Highly significant differences between IBS cases and controls regarding UBT and H P stool antigen test positivity were observed (P< 0.0001). There are significant differences between SIBO cases, and control regarding constipation, methane test positivity, hydrogen test positivity, and body mass index (P< 0.0001, < 0.0001, 0.023, and 0.045 respectively). There was a significant difference in stool antigen test positivity between SIBO and IBS, meaning that SIBO cases have more HP stool positivity than IBS cases. The production of methane in SIBO cases is higher and more significant than hydrogen production (P< 0.0001, P= 0.023 respectively).
Conclusion. Our study provides a new correlation between IBS, SIBO, and H. pylori infection. Moreover, while the relationship between H pylori and IBS is well known, our novel findings include; 1. The correlation between H pylori, IBS, and SIBO, 2. The correlation between SIBO and methane production, 3. SIBO cases have more HP stool positivity than IBS cases, 4. Based on the evidence presented herein linking urease to the pathogenesis of various functional gut disorders, we could inspire researchers to develop a new anti-urease treatment for functional gut disorders in the future.
Introduction
There is compelling evidence that microbe-host interactions in the intestinal tract underlie many human disorders, including disorders of gut-brain interactions (previously termed functional bowel disorders), such as irritable bowel syndrome (IBS). Small intestinal bacterial overgrowth (SIBO) has been recognized in patients with predisposing conditions causing intestinal stasis [1]. Helicobacter pylori infection and irritable bowel syndrome (IBS) negatively affect the quality of life. Some previous studies found that H. pylori infection should be positively associated with the risk of IBS, but others did not [2]. Examples of studies that confirmed that H. pylori infection is associated with an increased risk of IBS are Wang Z meta-analysis [3], Abdelrazak et al,[4], and Wang C et al [2]. Still, other studies denied this association such as Xiong F et al, [5], and Zhang J et al, [6]. The present study aims to clarify this association and to analyze whether H. pylori is associated with other functional dyspeptic disorders such as SIBO.
The microbe-host interaction in the intestinal tract underlies many human disorders, including disorders of gut-brain interactions, previously termed functional bowel disorders, such as irritable bowel syndrome (IBS) and small intestinal bacterial overgrowth (SIBO).
Functional Dyspepsia “FD” is a symptom complex characterized by postprandial upper abdominal discomfort or pain, early satiety, nausea, vomiting, abdominal distension, bloating, and anorexia in the absence of organic disease. Approximately 50% of patients with FD have motor disorders, such as antral hypomotility, impaired accommodation reflex, and gastric dysrhythmias. Studies using questionnaires showed that more than 75% of “FD” patients reported a relationship between aggravation of symptoms and ingestion of meals [7]. In a pilot interventional study, it was found that switching from a high-fiber diet to a low-fiber diet triggered FD-related symptoms and decreased small intestinal microbial diversity with increased small intestinal permeability [8]. FD patients often tend to self-diagnose with “food intolerances” and arbitrarily restrict their diet, solely based on their personal experience or anecdotal information from questionable sources. These improvised elimination diets are often nutritionally unbalanced and if prolonged, could cause nutritional deficiencies [9].
Helicobacter pylori. (H. pylori) is the ubiquitous, microaerophilic gram-negative spiral-shaped bacterium that affects up to 50% of the population worldwide with increased prevalence in developing countries with a low socio-economic status. It presents a substantial concern due to its link with gastric cancer, ranking as the third most common cause of global cancer-related mortality. H pylori is the most important cause of chronic or atrophic gastritis, peptic ulcer, gastric lymphoma, and gastric carcinoma. H. pylori infection is usually acquired in early childhood and persists without treatment [10,11]. Urease is one of the more abundantly produced proteins expressed by the pathogen, accounting for almost 15% of the total proteins of the bacterium. The production of urease is a characteristic feature of H. pylori and is widely used in its diagnosis [10].
Urease. Urease is a critical factor that facilitates bacterial colonization within the gastric mucosa; urease-negative mutants fail in colonizing the gastric mucosa at physiological pH levels as sufficiently as urease-positive H pylori strains do. Urease is a virulence factor found in various pathogenic bacteria [12]. Due to its enzymatic activity, urease has a toxic effect on human cells. The presence of ureolytic activity is an essential marker of several bacterial infections. Urease is also an immunogenic protein recognized by antibodies present in human sera. The presence of such antibodies relates to the progress of several long-lasting diseases, like rheumatoid arthritis, atherosclerosis, or urinary tract infections [13]. The polymorphisms in host genes encoding the immune effector proteins play essential roles in the inter-individual variations in clinical outcomes since they directly add to the susceptibility of an individual to H pylori and related diseases. Genetic polymorphism of H pylori virulence factors differs by geographic region, in which East-Asian-type cag A is known to be more virulent than Western-type [14].
Urease is produced by many different bacteria, fungi, and plants. Ureolytic bacteria may belong to symbiotic natural microflora or to pathogens. In facultative anaerobes from intestinal microflora, the level of this activity is diverse and species-characteristic. Ureolytic activity is often observed in pathogenic bacteria, such as pathogenic Staphylococcus, Helicobacter sp., E coli, Proteus, Klebsiella spp., and Pseudomonas sp. [15,16].
its link with gastric cancer, ranking as the third most common cause of global cancer-related mortality. H pylori is the most important cause of chronic or atrophic gastritis, peptic ulcer, gastric lymphoma, and gastric carcinoma. H pylori infection is usually acquired in early childhood and persists without treatment [10,11]. Urease is one of the more abundantly produced proteins expressed by the pathogen, accounting for almost 15% of the total proteins of the bacterium. The production of urease is a characteristic feature of H. pylori and is widely used in its diagnosis [10].
Irritable bowel syndrome (IBS) is a common chronic relapsing condition that affects up to 11% of the global population and 16.7–24.2% of the United States [17]. The understanding of IBS has changed since the release of the Rome IV diagnosis in 2016. With the upcoming Rome V revision, it is necessary to review the results of IBS research in recent years. It mainly affects young and female individuals, and it tends to overlap with other functional gastrointestinal diseases ‘FGIDs’ [18]. Prevalence varies significantly between countries because of differences in food, culture, and diagnosis. The Rome Foundation Global Study [19] coverage across the country reported that the overall prevalence of IBS was 3.8% in Rome IV and 10.1% in Rome III. The Rome IV criteria, based on symptoms that have changed in dynasty, suggest that the pathogenesis of IBS is associated with gut-brain interactions, which may be an overlapping pathogenesis between FGIDs. The pathophysiology of IBS is complex and the role of risk factors such as genetics, diet, and microbiome might operate differently, depending on geography [20]. Studies of IBS-related dietary interventions, such as LFD, special diets for IBS-C, and foods with gastrointestinal allergies, as well as the gut microenvironment and the brain-gut axis, are the hot spots of research on gut inflammation and the gut barrier [21].
Small intestinal bacterial overgrowth: The definition of SIBO as a clinical entity lacks precision and consistency; it is a term generally applied to a clinical disorder in which symptoms, clinical signs, and/or laboratory abnormalities are attributed to changes in the numbers of bacteria or in the composition of the bacterial population in the small intestine [21]. It is defined as an increase in the bacterial content of the small intestine above normal values “100000 cells per mL” [22,23,24]. The exact prevalence of SIBO in the general population is unknown. Most authors report rates between 2.5 and 22% and emphasize that the prevalence increases with age and in populations with comorbidities [24,25]. IBS and SIBO symptoms are nonspecific and overlap. Recent realization that SIBO may be associated with symptoms of IBS, led to a paradigm shift in understanding the pathogenesis of this condition [26]. The human gut microbiota creates a complex microbial ecosystem characterized by its high population density, wide diversity, and complex interactions. Any imbalance of the intestinal microbiome, whether qualitative or quantitative, may have serious consequences on human health, including SIBO [27]. It is worth noting that forms of SIBO differ depending on the predominant species of bacteria colonizing the small intestine, and these depend on their origin. There are two well-established types: upper aerodigestive tract (UAT) SIBO and coliform SIBO [28]. UAT-SIBO is caused predominantly by oral cavity bacteria, including Prevotella sp., and Streptococcus viridans, whereas coliform SIBO is caused predominantly by bacteria from the distal segments of the gastrointestinal tract, such as E coli, Klebsiella pneumoniae, Proteus mirabilis, or Enterococcus sp. [24,28]. Despite the distinction between the two types, their importance in clinical practice is limited due to similar symptoms and treatment [24]. An increase in the abundance of Enterobacteriaceae is the main alteration to the gut microbiome that correlates with SIBO diagnosis and symptom severity. The enhancement of specific gas-producing pathways has been demonstrated in SIBO [25]. These pathways lead to excessive gas formation in the small intestine, bloating, and pain in the umbilical region, which may be accompanied by malabsorption, malnutrition, and osmotic diarrhea. The leading causes of SIBO are dysfunctional small intestinal movement and delayed orocecal transit time (OCTT). The pH changes make it easier for bacteria from other areas to grow in the small intestine and allow different types of bacteria to thrive, for example, due to the use of proton pump inhibitors (PPI) or after gastric surgery, and reflux of the colon contents into the small intestine due to ileocecal valve dysfunction [22,29]. Interest in SIBO is increasing, as evidenced by the continuous growth in publications on this topic indexed in PubMed (Fig. 1). The main diagnostic methods are lactulose and glucose breath tests (LBT, GBT), as well as jejunal culture quantification [30,31]. While SIBO was initially associated with hydrogen release, it is now accepted that SIBO can also accompany the formation of methane [32].
Fig. 1, indicates the number of publications indexed by PubMed on small intestinal bacterial overgrowth by year [33].
Methanogens and intestinal gases. Archaea are a group of single-celled microorganisms with distinct characteristics. Archaea have emerged as important components of the human microbiome. Despite their potential importance in human health and disease, archaea are relatively less studied than other members of the microbiome such as bacteria and fungi. The human-associated archaeal communities exhibit diverse patterns like those of bacteria; notably are the predominant ammonia-oxidizing Nitrososphaeria on the skin, and methane-producing (methanogenic) Archaea in the urogenital and gastrointestinal tracts [34]. Methanogenic archaea (methanogens) are ubiquitously present in anaerobic environments, such as digestive tracts, paddy fields, and aquatic sediments, and play an important role in anaerobic degradation of organic matter and the global cycle of carbon [35].
Methanogens produce methane gas and can overgrow in the small intestine or the large intestine. So, the name Intestinal Methanogen Overgrowth is more fitting than SIBO. While methane is associated with constipation, hydrogen gas produced with SIBO is associated with diarrhea. Methane gas slows transit time while hydrogen speeds it up. So, when you have too many methane producers, you get constipation [36,37,38].
Methanogens have been associated with dysbiosis as in vaginosis, urinary tract infections, and anaerobe abscesses of the brain, and muscles. Additionally, anaerobic infections in periodontitis, periimplantitis, refractory sinusitis, and endocarditis [39]. The possible ways of the methanogens’ acquisition in humans may be contact with animals or consumption of milk dairy products of certain animals, particularly cows since a recent study demonstrated an association between the acquisition of M. smithii in children and the consumption of dairy products [40].
Although gut microbiota produces many volatile compounds as part of its metabolism (including short-chain fatty acids), the gases that make up the majority of this volume are hydrogen (H2), carbon dioxide (CO2), and methane (CH4). These gases that contribute to more than 99 % of the intestinal gas volume are odorless [41]. The unpleasant odor associated with intestinal gases comprises less than 1 % of the total intestinal gas volume and it is the result of trace gases such as hydrogen sulfide (H2S), and dimethyl sulfide [(CH3)2S] as well as other volatile compounds such as short chain fatty acids [42]. The short-chain fatty acids (SCFAs) butyrate, propionate, and acetate are microbial metabolites, their availability in the gut and other organs is determined by environmental factors, such as diet and use of antibiotics. Intestinal SCFAs also affect immunity at extra-intestinal sites, such as the liver, the lung, the reproductive tract, and the brain. They have been implicated in various disorders, including infections, intestinal inflammation, autoimmunity, food allergies, asthma, and responses to cancer therapies [43]. Human gut microorganisms form complex microbial communities that depend on one another to harvest nutrients and energy to survive. Metabolites produced by one strain in the community may be further utilized by another. The metabolites released by microbiotas differ across individuals [44]. Profiling of intestinal gases and their responses to dietary changes can reveal the products and functions of gut microbiota and their influence on human health. Profiles of intestinal gases are predominantly influenced by the composition of the luminal microbiota and by consumed dietary substrates. Dietary manipulations readily alter intestinal gas production and composition and are therefore considered attractive tools in managing patients with gas-associated gastrointestinal disorders [45,42].
Methane. Recent ACG guidelines proposed a new term to describe increased levels of methane gas production that is; the intestinal methanogen overgrowth (IMO). Furthermore, methanogens may overpopulate either the colon or the small intestine [46]. A level of methane gas level ≥10 ppm observed at any time during the breath testing (including at baseline in a fasting patient) is considered a positive IMO test result [47,48]. Multiple studies have identified that higher methane levels are positively associated with constipation and are inversely associated with diarrheal disorders [49,50,51], and methane levels correlate with slower intestinal transit times [52]. Methane-predominant SIBO/IMO is more prevalent in patients with constipation-predominant IBS [46].
Methods.
Per our knowledge, this is the first study to uncover the correlation of urease enzyme to the pathogenesis of IBS, SIBO, and other functional gut disorders. It is important to note that due to the possible extraneous variables not controlled for in the study design, we had selected matching participants based on specific socio-demographic characteristics, such as age, sex, etc., and were statistically controlled for these variables in the analysis. Thus, the results of this study can suggest a possible association and a cause-effect.
Study design. A randomized case-control study in which the selection of participants is equivalent to those matching criteria based on previous knowledge of an association with the outcome of interest.
Setting & Participants. Participants were recruited from different geographic regions in Egypt. The study approval was received from the administration of Hurgada, Marsa Alam, and Nasser Institute Hospitals in Cairo; and from Abu Hareez-sharkia Governorate Hospital, Egypt. Routine consents for laboratory diagnosis were implemented for all cases according to hospital regulations. The study protocol conformed to the ethical guidelines of the “1975 Declaration of Helsinki”.
Variables. Cases and controls were matched for any potential confounder and were recruited from the same population and locality. Selected parameters included age, sex, urbanization, social status, and comorbidities showed a close balance between the cohorts. All participants were seen for examination, which included vitals, weight, height, body mass index (BMI), and demographic variables gathered by the questionnaire including age, sex, occupation, smoking status, and family and past histories.
Data sources/ measurement. Sources for cases include patient rosters at medical facilities. The comparison group ("controls") was representative of the population that is the same source of cases and sampled them in a way that is independent of the exposure to gut disorders. Both groups were matched for socio-demographic characteristics. Eligible cases were enrolled from outpatient clinics as well as inpatient admissions after informed consent was obtained. Records of information were collected to verify inclusion criteria, medications given, clinical review, family and past histories as well as test results.
Sample size & Bias. Cases were recruited from hospitals from August 2023 to July 2024. A total of 225 cases were studied; 125 were IBS (44 males and 81 females) and 100 were SIBO (72 males, 28 females) compared to 100 controls (37 males and 63 females), all aged 18 to 60 years. It doesn’t matter the difference in number between cases and control groups because it is the percentage of occurrence of the event and the comparative association of the event outcome relevant to the groups which are, reliable indicators of this case-control study.
Quantitative variables. IBS cases were assorted according to Rome IV criteria. Likewise, SIBO cases were selected according to SIBO diagnostic criteria.
Analytical statistics. All analyses were performed using SPSS, version 18.0 (SPSS Inc., Armonk, NY, USA). The demographic characteristics of cases and controls were compared using the Fisher exact test, adjusted Odds Ratios, and 95% Confidence Intervals (CIs) calculation. Continuous variables (age, BMI) were presented as means with standard deviations. Categorical variables (sex, smoking, living area) were presented as absolute numbers and percentages. T test or one-way analysis of variance was used to compare continuous variables and Fisher’s exact test to assess the relationship between categorical variables. A statistically significant result was defined as a P value less than 0.05.
IBS Diagnostic Criteria. The criteria for a diagnosis of irritable bowel syndrome (IBS) require that the person has experienced recurrent abdominal pain on average at least one day a week in the last three months, with an onset of symptoms at least six months prior. Pain must be associated with two or more of the following:
• Related to defecation
• Associated with a change in the frequency of stool
• Associated with a change in the appearance of stool
Because IBS can have variable symptoms, it is classified into subtypes according to the predominant habit, ie constipation, diarrhea, or mixed; (IBS-C). (IBS-D), (IBS-M)
These criteria should be fulfilled for the last 3 months with symptom onset at least 6 months prior to diagnosis, modified from Rome IV [53]. Per our knowledge, this is the first study to uncover the correlation of urease enzyme to the pathogenesis of IBS, SIBO, and other functional gut disorders. It is important to note that due to the possible extraneous variables not controlled for in the study design, we had selected matching participants based on specific socio-demographic characteristics, such as age, sex, etc., and were statistically controlled for these variables in the analysis. Thus, the results of this study can suggest a possible association and a cause-effect.
Study design. A randomized case-control study in which the selection of participants is equivalent to those matching criteria based on previous knowledge of an association with the outcome of interest.
Quantitative variables. IBS cases were assorted according to Rome IV criteria. Likewise, SIBO cases were selected according to SIBO diagnostic criteria.
Analytical statistics. All analyses were performed using SPSS, version 18.0 (SPSS Inc., Armonk, NY, USA). The demographic characteristics of cases and controls were compared using the Fisher exact test, adjusted Odds Ratios, and 95% Confidence Intervals (CIs) calculation. Continuous variables (age, BMI) were presented as means with standard deviations. Categorical variables (sex, smoking, living area) were presented as absolute numbers and percentages. T test or one-way analysis of variance was used to compare continuous variables and Fisher’s exact test to assess the relationship between categorical variables. A statistically significant result was defined as a P value less than 0.05.
IBS Diagnostic Criteria. The criteria for a diagnosis of irritable bowel syndrome (IBS) require that the person has experienced recurrent abdominal pain on average at least one day a week in the last three months, with an onset of symptoms at least six months prior. Pain must be associated with two or more of the following:
• Related to defecation
• Associated with a change in the frequency of stool
• Associated with a change in the appearance of stool
Because IBS can have variable symptoms, it is classified into subtypes according to the predominant habit, i, e constipation, diarrhea, or mixed; (IBS-C). (IBS-D), (IBS-M)
These criteria should be fulfilled for the last 3 months with symptom onset at least 6 months prior to diagnosis, modified from Rome IV [53].
Patients. The exclusion criteria were (1) use of any antibiotics or probiotics within a month; (2) use of promotility drugs and laxatives within a week; and (3) patients with digestive diseases (liver cirrhosis, inflammatory bowel disease, chronic pancreatitis, and gastrointestinal tumors), severe systemic disease, or history of abdominal surgery.
Diagnostic Criteria of SIBO. Patients meeting the following positive criteria for hydrogen/methane breath test were considered positive for SIBO. Hydrogen test positive: (1) fasting hydrogen level ≥20 parts per million (ppm); or (2) a ≥20 ppm rise in hydrogen by 90 minutes. Methane test positive: methane levels ≥10 ppm at any test point [54].
Lactulose Hydrogen‐Methane Breath Test. The Hydrogen‐methane breath test was used as a noninvasive test for SIBO. All subjects fasted for 8 to 12 hours. Fermentable foods, such as dairy products, soy products, and fiber‐rich foods were avoided on the day before the test. Smoking and physical activity were prohibited, and the oral cavity was kept clean on the test day. After preparation, the subjects held their breath for at least 10 seconds and blew into the collection bag, avoiding ventilation throughout the process. Then, 10 g of lactulose was taken orally, and the gas collection step was repeated every 30 minutes. The test lasted for 90 minutes, and four gas bags were collected. After collecting exhaled gases, the concentration of both hydrogen and methane was measured using the Nano Coulomb Breath Analyzer (Sunvou Biotechnology Co., Ltd, Wuxi, Jiangsu, China).
Urea Breath Test. The 13C urea breath test was performed using 75 mg urea (UREA 13C breath test Heliforce kit, Beijing Richen-Force Science & Technology Co. Ltd., Beijing, China). The test was performed according to the manufacturer’s instructions, briefly as follows:
A basal breath sample was obtained by asking the patient to take a deep breath and hold it for 10 seconds before blowing the exhaled gas into a specific bag at zero time. After this, the patient was asked to drink the reagent which contains urea attached to a 13C carbon atom in 90 mL of water. Then, 30 min later, the patient was similarly asked to give a breath sample again, which was collected into a new specified bag.
Samples were analyzed by infrared spectrophotometer (IR force-200 Infrared Spectrometer, Beijing Richen-Force Science & Technology Co. Ltd., Beijing, China) to measure the 13C isotopic abundance of the 30 min and zero-minute breath samples as per the manufacturer’s instructions. The final measured value by the spectrophotometer is called delta over base DOB, and when the DOB value is ≥4.0 ± 0.4, it is considered positive for H. pylori.
Stool Antigen Testing for H. pylori. Stool samples were processed immediately using stool antigen cards (Abon Biopharm hangzhou Co. Ltd., Hanghazue, China) according to the manufacturer’s instructions.
Statistical Analysis. Data generated from the study are tabulated and uploaded to the Statistical Package for Social Sciences (SPSS version 22, IBM Corp., Armonk, NY, USA). The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for the 13C-UBT and H. pylori stool antigen tests were calculated.
Results.
This study was designed to investigate the interrelation between H pylori, Methanogens, food, and functional dyspeptic Syndromes. Among 375 patients who were initially recruited into the study, 13 patients failed to complete the sample collection, 8 patients received antibiotics treatment and 29 patients falling short of inclusion criteria were excluded. Eventually, 325 consecutive patients were selected in this study.
Participants. A total of 225 cases with functional dyspeptic disorders compared to 100 controls were studied. Our findings derived from matched data indicated that there is a strong association between urease enzyme mainly related to H pylori, and increased incidence of functional dyspeptic disorders, food, and methanogenic bacteria.
Descriptive data. Table 1 displays the comparison of sociodemographic variables among IBS and control groups. Notably, there are highly significant differences between IBS cases and controls regarding UBT and H P stool antigen test positivity. Table 2 shows demographic characteristics and sociodemographic variables among SIBO and control groups. There are significant differences between SIBO cases and control regarding constipation, methane test positivity, hydrogen test positivity, and body mass index. It is noted that the difference is higher and more significant in both constipation and methane positivity. Table 3 presents analyzed and compared laboratory tests among cases and controls. Table 4 further analyzed, and compared laboratory tests among diseased groups. There was a significant difference in stool antigen test positivity between SIBO and IBS, meaning that SIBO cases have more HP stool positivity than IBS cases. Table 5 shows the comparison of methane and hydrogen breath tests between the SIBO and control groups. The production of methane in SIBO cases is higher and more significant than hydrogen production.
Discussion.
The main purpose of this study is to evaluate and compare functional dyspeptic disorders (SIBO & IBS) among two adult populations and their correlation with H. pylori and methanogenic organisms in various regions in Egypt. Functional dyspepsia is a complex and multifaceted disorder that is studied by a broad range of disciplines across clinical and microbiological sciences. To address the medical challenges that range from new laboratory technologies, modes of interaction, overlapping disease symptoms, and sociodemographic and climate change, we should explore the various aspects of gut environmental framework and regulation. These contributions highlight how achieving progress in each discipline will require incorporating insights and methods from others to break down disciplinary silos.
Genuine progress in understanding functional gut disorders related to microbiota and food can only be achieved through a multidisciplinary community effort to share their views on new directions in their disciplines. Their works provide rich insight into the future of research on gastrointestinal mysterious disorders. Some previous studies found that H. pylori infection should be positively associated with the risk of IBS, but others did not.
The complex interplay between H. pylori and its human host has spurred intensive research efforts to elucidate its pathogenesis, develop accurate diagnostic methods, and plan for adequate treatment strategies. Furthermore, the microbiome impact has been shown to extend beyond the gut and found to be in link to autoimmune disorders, cardiovascular diseases, and metabolic syndromes that have been explored by many studies. The layer of complexity we have uncovered is nothing short of astonishing.
Although IBS studied for a long time, it still seems to be an unexplored disorder with various proposed etiologies. Several methodological discrepancies may explain some of the observed different presentations. It is concluded that a pooled global prevalence of IBS is unlikely to be meaningful and that future research should focus more on microbiota regionalization which will demonstrate the distinguishing different patterns of microbiota within various regions of the world.
The symptoms of SIBO overlap with many other gastrointestinal conditions. SIBO, might not be the first thing the healthcare provider suspects. The prevalence of SIBO is not well-defined. It is in part due to variability in presenting symptoms and diagnostic methods in addition to similar symptoms common to other conditions. A meta-analysis by Gurusamy et al suggested a link between FD and SIBO. The quality of evidence can be largely attributed to the type of breath test for SIBO diagnosis and clinical heterogeneity. Substantial heterogeneity was found in studies using the lactulose breath test but not in studies using GBT [55]. Interestingly, bacterial overgrowth can lead to a false positive H. pylori diagnosis using urea-based testing given the presence of urease-positive bacterial strains [56].
It is evident from our results that urease enzyme is the metabolic mechanism of methane production from methanogenic bacteria. This notion could be explained by the hydrolytic effects of urease to produce carbon dioxide and ammonia. Our conclusion was affirmed and extensively studied over the past few decades for biomethane production from converting CO2 into chemical products by using microbial methanogenesis cells (MMCs). It was found that CO2 is utilized by intestinal methanogenic bacteria to produce methane [57]. Additionally, catholyte pH control and CO2 supply methods were critical operating factors impacting microbial methanogenesis cells ‘MMCs’ [58]. Furthermore, studies indicate that the impedance of fuel cells increases significantly due to ammonia through the catalytic reaction of hydrogen and oxygen during the experimental generation of energy [59]. It was evidenced that the traditional anaerobic ammonia oxidation (anammox), which refers to the anammox bacteria (AnAOB) used ammonia as an electron donor and nitrite as an electron acceptor to generate dinitrogen ‘NH4+ + NO2- → N2’ [60]. Moreover, research on methane production using ammonia as the sole electron donor also indicated that these methanogenic processes often produce hydrogen first, which is then utilized by hydrogenotrophic methanogens to produce methane [61].
In short. Urea hydrolysis to ammonia and CO2 by urease could affect microbial metabolic methanogenesis and methane production through various mechanisms.
Our results show that there are significant associations between SIBO, constipation, methane test positivity, and hydrogen test positivity. The difference is higher and more significant in both constipation and methane positivity which indicates that methane production is primarily correlated with constipation. As seen from the study results, there is a significant association of SIBO with increased body mass index (BMI). This correlation was evaluated by studies done by Kalantar et al [62], and Basseri et al [63]. It has been suggested that by slowing down the transit, the time for nutrient absorption is lengthened, which together with boosted levels of methanogenic microorganisms in the intestines, could lead to an increased weight gain process and thus the development of obesity. Moreover, we dive further into mechanisms of obesity caused by methanogenic bacteria. We figure out studies such as Laverdure et al [64] which indicated that alterations in the methanogens occurring in obesity may play a vital role in directly enhancing GLP-1 secretion, and that methane can directly stimulate the secretion of GLP-1.
Methane-induced metabolic disorders.
The study results indicate the marked interrelation and pathogenic interplay between H pylori urease toxic metabolites (CO2, NH3) and methane production. The overall metabolic disorders seen in IBS, SIBO, and other functional gut disorders may originate from the key enzyme urease and its impact on methane-producing organisms. It was demonstrated that the increasing methane production is due to anoxic environments and the change in methane concentration might itself be a signal that an organism should temporarily stop consuming energy. With its property of small size, methane could penetrate nuclear membranes, mitochondria, ER, and other cellular components to exert protection under hypoxic conditions [65]. Further data suggest that endogenously produced methane can influence metabolism and thus energy homeostasis. More importantly, higher methane concentrations in the GI tract can significantly slow transit time [66]. Yu Song et al study suggested that bacterial translocation resulting from disruption of the intestinal barrier causes SIBO. Then SIBO leads to endotoxins, microbial components, and metabolites that enter the intestinal mesenteric artery, thus activating systemic inflammatory and immunological responses, eventually causing hypertrophy, apoptosis, and fibrosis of cardiomyocytes [67]. SIBO has also been shown to be closely associated with many parenteral diseases, such as deep vein thrombosis [68], Parkinson’s disease [69] diabetes mellitus [70], atherosclerosis [71], and coronary artery disease [72].
Singh et al reviewed data on gut dysbiosis and interactions involved in the pathogenesis of gut dysmotility and metabolic syndrome (Met S) conditions. They found “Met S” including IBS, functional dyspepsia (FD), and type 2 diabetes mellitus (T2DM) are greatly influenced by the gut, thereby metabolic disorders could begin there [73].
In this respect, H pylori infection induces GIT dysmotility. Likewise, H. pylori-associated Met S conditions are related to dysmotility-stimulated gastrointestinal microbial overgrowth, potentially leading to bacteremia with systemic conditions. “Met S” occurs commonly in patients with SIBO [74]. Interestingly, ammonia produced by H pylori and other urease organisms is considered tonic to the gut smooth muscles, as described in the literature [75, 76]. Therefore, it can cause multiple colonic spasms leading to colon re-absorptive error with recurrent abdominal pain, vomiting, and anorexia, in addition to mental manifestations such as irritability, depression, and anxiety. These highly variable clinical presentations are characteristics of IBS and SIBO.
Gut Anaerobic Organisms and Methane. The study implications highlight the important focus on the complexities of bacterial communities and their interactive metabolic response to the host. This is true particularly, in response to diet and related digestive factors such as gastric acid and bacterial virulence factors including their metabolic end products. This perspective has some intriguing reorganization for understanding the gut ecosystem. Studies indicated that the obligatory anaerobic microbiota is dominantly inhabiting the gut, thus preventing the expansion of facultative aerobic bacteria in part by limiting the production of oxygen and nitrate by the host. It is estimated that anaerobic bacteria outnumber aerobic and facultative anaerobic bacteria by 100- to 1,000-fold [77]. It is reported that anaerobic bacteria differ from aerobic bacteria in their oxygen requirement. Oxygen is toxic to anaerobes which can be explained by the absence of catalase, superoxide dismutase, and peroxidase enzymes in anaerobes [78].
The study results illustrate the role of the gut environmental system in promoting the dominance of anaerobic bacterial microbiota at the expense of aerobic bacteria because of the relative lower oxygen and pH inside the GIT environment. Morais et al. reported that lower oxygen significantly increases methane production rate (MPR) from acetate or H2/CO2 [79]. Methane production involves the reduction of acetate or carbon dioxide in a microaerophilic or anaerobic environment under the catalytic actions of methyl coenzyme M to generate methane [80]. Diederich et al. observed that lower pH enabled the stabilization of aldehydes [81]. Interestingly, the aldehyde “Acetaldehyde” results most likely from the hydration of acetylene. Moreover, acetylene is a gaseous compound that is formed through the process of methane photolysis [82]. Taken together, it is concluded that aldehyde is the product of methane, and CO2 (the product of hydrolysis of urea by urease) is utilized by the intestinal methanogenic bacteria to produce methane [58].
These findings shed light on the significance of metabolic interactions in the dynamics of microbial communities, particularly in determining the outcome of pathogenic invasions or microbial interventions and focusing on microbiome modification.
To delve deeper into the environmental adaptability of microbiota within the gut ecosystem influenced by bacterial metabolic activities, we should address the mystery of the metabolic cascade of anaerobic-aerobic balanced interplay as elucidated by recent studies. This knowledge of the highly dynamic nature of the anaerobic-aerobic microbial community can help us to get valuable insights into the environment of microbiota and gut microbial metabolic activities. In this context, we will provide a novel treatment for functional disorders “such as IBS and SIBO” that seemingly result from the disturbed anaerobic-aerobic equilibrium. Metabolic interactions were further demonstrated to strongly affect colonization and competitive interactions of anaerobic-aerobic microbiota. Our finding-based notion suggests the use of oxygen-producing medications or reactions integrated with aerobic organisms to counteract the risks of anaerobic bacteria, which seem involved in the causation of diseases related to their metabolic products, CO2, CH4, NH3, etc.
Consistent with our concept of the potential use of oxygen in treating many gut disorders, we encourage ongoing research and collaborative efforts to further foster, and research advances in the metabolism of gut microbiota. Julius et al, reported that anaerobic gut bacteria demonstrate the highest degree of oxygen sensitivity. Furthermore, although many bacteria can survive oxygen by mechanisms such as sporulation; oxygen-free conditions are required for the anaerobic bacteria to grow [83]. The main stages of anaerobic digestion include the sequential breakdown of organic matter through hydrolysis, acidogenesis, acetogenesis, and methanogenesis. Substrate degradation, electron transfer, and energy conservation could determine the performance, stability, and resilience of anaerobic digestion [84]. So far, varied forms of anaerobes have been shown to produce carbon dioxide, hydrogen, and methane from different types of wastes, such as a carbon source, and CO2 is further made from volatile fatty acids through acetogenesis [85].
The Microbiota -Dietary metabolic pathway: To dig deeper into understanding gut ecological systems. We provide innovative solutions to longstanding challenges of the highly dynamic nature of the microbial community and drive the equilibrium status towards the dominance of aerobic bacteria, we propose the implementation of microbes found in foods and involved in human gut health that harbor O2-resistant Bacillus and Brevibacillus based-probiotics (Brevibacillus massiliensis strain phR) which are obligately aerobic microbe that was isolated from human feces. The potential advantages of these microbes arise from the fact that they readily take up tungsten (W), a metal previously associated only with anaerobes. The "W" incorporated into an oxidoreductase enzyme (BmWOR) could exhibit high aldehyde-oxidizing activity with very high affinities for aldehydes common in the human gut and in cooked foods, including furfural, propionaldehyde, and benzaldehyde, suggesting that they play a key role in their detoxification. These suggestions maximize the benefits of using aerobic bacteria to overcome anaerobes and their essential metabolic precursors. Thorgersen et al explored the role of aerobic “Brevibacillus massiliensis, strain phR” in the detoxification of anaerobic bacteria, and reported that anaerobic enzymes are rapidly inactivated, often within seconds, upon exposure to oxygen [86]. One of the two WORs was the so-called electron bifurcating enzyme that simultaneously reduced NAD and FAD and it was hypothesized that such a strategy enabled the removal of extremely low concentrations of aldehydes in the gut environment, even in the presence of high concentrations of acids [87].
In summary. To overcome the dominance of anaerobic bacteria and the risk of their metabolic byproducts, we employ the concept of oxygen utilization and exploiting certain beneficial aerobic bacterial strains (Brevibacillus massiliensis, strain phR) for the treatment of many gut disorders through detoxification reactions directed against anaerobic bacteria.
To reveal the potential significance of food dietary interventions such as the advice of certain diet for IBS-C, and gastrointestinal allergies. We should consider the study results which document wide variations in the prevalence of functional gut disorders among geographic localities. They concluded that functional gut disorders were attributed to multifactorial etiologies, such as type of food, biodiversity, and allergic pollution monitoring, contributing to more understanding of microbiota ecological systems. Moreover, the functional disorder named traveler’s diarrhea was found to be linked to the food and microbiota characterizing specific cultural and geographical areas. Examples of studies that maximize our conclusive findings include Huang et al [19], Black et al [20], Skrzydło-Radomańska et al, [24], and Sharabi et al [25].
Further studies confirmed that dietary manipulations readily alter intestinal gas production and composition, energy metabolism, gut transit regulation, immunity, paracrine and eccrine regulation, and bacterial proliferation. These are considered attractive tools for managing patients with gas-associated gastrointestinal disorders [42,43,45]. It was found that the high-fat diet caused a significant increase in both GLP-1 secretion and fecal methanogen content. There was a direct correlation between GLP-1 secretion response and fecal methanogen levels [64]. Furthermore, Zheng et al stated that GLP-1 acts on the GLP-1 receptors to activate multiple intracellular signaling pathways, including the cAMP/PKA signaling pathways, anti-inflammatory pathways, and anti-oxidative stress responses. These pathways are crucial in wide-ranging therapeutic effects beyond metabolic diseases [88]. The mechanism explaining methane's action on metabolic health may be through direct action on the GI endocrine system. Studies demonstrated that in addition to housing methanogens, the GI tract is also considered home to various metabolic hormone-secreting cells, known collectively as the enteroendocrine system. Specifically, glucagon-like peptide-1 (GLP-1) is secreted from the L cells of the lower GI epithelium and plays a vital role in post-meal insulin secretion and appetite suppression. Like the L cells, methanogens are most abundant in the lower small intestine and colon. Recently, bacterial metabolites including short-chain fatty acids were shown to stimulate GLP-1 secretion. Interestingly, these metabolites can also be methane precursors [88,89,64].
To sum it all up, the wide variations in the prevalence of functional gut disorders and the overlapping symptoms among various geographic localities all over the world are attributed to multifactorial etiologies, such as type of food, biodiversity, medications (antibiotics and PPIs) and allergic pollution, which are contributing to the diversity of gut microenvironmental systems. Understanding functional gut disorders related to microbiota and food indicated that bacterial metabolites such as ureases are the etiological key enzymes of various functional gut disorders. Moreover, other metabolites including short-chain fatty acids were shown to stimulate GLP-1 secretion and can be methane precursors. This clarifies the role of the enteroendocrine system as the mediator of metabolic disorders.
Conclusion. The urease enzyme is the primary etiologic mechanism of functional dyspeptic disorders including methane production by methanogenic bacteria. This could be explained by the hydrolytic effects of urease to produce carbon dioxide and ammonia. CO2 is used by intestinal methanogenic bacteria to produce methane. Additionally, catholyte pH control and CO2 supply were critical operating factors impacting microbial methanogenesis. Functional dyspepsia is a complex and multifaceted disorder with medical challenges that range from new laboratory technologies, modes of interaction, overlapping disease symptoms, and sociodemographic changes. Knowledge of the highly dynamic nature of the anaerobic-aerobic microbial community would give valuable insights into the environmental adaptability of microbiota within the gut ecosystems influenced by bacterial metabolic activities. Metabolic interactions were demonstrated to influence colonization aspects and competitive interactions of the gut microbiome. In this context, we will provide a novel treatment of functional disorders resulting from the disturbed anaerobic-aerobic interplay and through detoxification reactions directed against anaerobic bacteria.
Future directives.
1. Our finding-based notion suggests the use of oxygen-producing medications or reactions integrated with aerobic organisms to counteract the risks of anaerobic bacteria which is seemingly involved in the causation of diseases related to their metabolic products.
2. Consistent with our concept of the potential use of oxygen in treating many gut disorders, we encourage ongoing research and collaborative efforts that need to be firmly addressed to elucidate further advances in the metabolism of gut microbiota. Furthermore, we suggest future directions that foster multidisciplinary sustainable practices in this field.
3. We provide innovative solutions to longstanding challenges of the highly dynamic nature of the microbial community and drive the equilibrium status towards the dominance of aerobic bacteria. This inspired our rigorous medical sense to propose microbes found in foods and involved in human gut health that harbor O2-resistant Bacillus and Brevibacillus-based probiotics. Understanding the gut ecological systems made it possible to maximize the benefits of using aerobic bacteria to overcome the potential risks of anaerobes and to detoxify their essential metabolic precursors.
Conclusion. The urease enzyme is the primary etiologic mechanism of functional dyspeptic disorders including methane production by methanogenic bacteria. This could be explained by the hydrolytic effects of urease to produce carbon dioxide and ammonia. CO2 is used by intestinal methanogenic bacteria to produce methane. Additionally, catholyte pH control and CO2 supply were critical operating factors impacting microbial methanogenesis. Functional dyspepsia is a complex and multifaceted disorder with medical challenges that range from new laboratory technologies, modes of interaction, overlapping disease symptoms, and sociodemographic changes. Knowledge of the highly dynamic nature of the anaerobic-aerobic microbial community would give valuable insights into the environmental adaptability of microbiota within the gut ecosystems influenced by bacterial metabolic activities. Metabolic interactions were demonstrated to influence colonization aspects and competitive interactions of the gut microbiome. In this context, we will provide a novel treatment of functional disorders resulting from the disturbed anaerobic-aerobic interplay and through detoxification reactions directed against anaerobic bacteria.
Future directives.
1. Our finding-based notion suggests the use of oxygen-producing medications or reactions integrated with aerobic organisms to counteract the risks of anaerobic bacteria which is seemingly involved in the causation of diseases related to their metabolic products.
2. Consistent with our concept of the potential use of oxygen in treating many gut disorders, we encourage ongoing research and collaborative efforts that need to be firmly addressed to elucidate further advances in the metabolism of gut microbiota. Furthermore, we suggest future directions that foster multidisciplinary sustainable practices in this field.
3. We provide innovative solutions to longstanding challenges of the highly dynamic nature of the microbial community and drive the equilibrium status towards the dominance of aerobic bacteria. This inspired our rigorous medical sense to propose microbes found in foods and involved in human gut health that harbor O2-resistant Bacillus and Brevibacillus-based probiotics. Understanding of gut ecological systems made it possible to maximize the benefits of using aerobic bacteria to overcome the potential risks of anaerobes and to detoxify their essential metabolic precursors.
4. Hopefully, the evidence presented herein linking urease to the pathogenesis of various functional gut disorders will encourage investigators of clinical trials to find new methods to overcome the undesired effects of antibodies against urease. Future studies on urease inhibitors should aim to develop efficient new generations with the least possible side effects. It will uncover new avenues to eradicate and abolish urease, as it is considered the cornerstone of the pathogenetic mechanism of most bowel disorders.
Abbreviations.
FD = Functional Dyspepsia.
GIT = gastrointestinal tract
Oro cecal transit time; is defined as the time taken from ingesting lactulose to the first sustained rise of hydrogen or methane or both in breath ≥12 ppm above the base line value.
AGA = American Gastroenterological Association.
GLP-1 = glucagon-like peptide-1
GLP-1R = glucagon-like peptide-1 receptor.
BmWOR = Tungsten incorporated oxidoreductase enzyme.
LFD = low-fermentable oligo-, di-, and monosaccharides and polyols (FODMAP) diet.
NAD = Nicotinamide adenine dinucleotide
FAD = Flavin Adenine Dinucleotide.
Disclosure of conflict of interest. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors declare no conflict of interest.
Funding. The authors assure that they never received funding assistance from either individuals or organizations.
Table 1. Comparison of sociodemographic variables among IBS, and control groups.
|
Characteristics |
All IBS |
IBS-C |
IBS-D |
IBS-M (N = 16) |
Control P value |
|
Mean age (years [±SD)] |
43 (±14) |
41 (±16) |
40 (±16) |
40(±16) |
42(±15) 0.607
|
|
Gender (%): |
|
||||
|
Male |
44 |
23 |
13 |
8 |
37 |
|
Female |
81 |
44 |
25 |
12 |
63 |
|
Marital status (%): * |
|
||||
|
Married |
50 |
28 |
14 |
8 |
42 |
|
Single |
48 |
26 |
12 |
10 |
45 |
|
Divorced |
20 |
10 |
6 |
4 |
13 |
|
Widowed |
7 |
5 |
2 |
0 |
0 |
|
Employment (%): * |
|
||||
|
Unemployed |
34 |
18 |
10 |
6 |
25 |
|
Full time |
63 |
41 |
17 |
5 |
48 |
|
Part time |
28 |
12 |
9 |
7 |
27 |
|
Body mass index kg/m2 |
25.2±4.2 |
24.8±4.1 0.474 |
|||
|
Smoking |
47 |
25 |
10 |
12 |
35 0.687 |
|
Urea Breath Test +ve |
87(69.6%) |
9(9.0%) < 0.0001 |
|||
|
H P Stool Ag Test +ve |
53(42.4%) |
8(8.0%) < 0.0001 |
|||
No significant differences between IBS and control group regarding body mass index and smoking. There are highly significant differences between IBS cases and controls regarding UBT and H P stool antigen tests.
The logistic regression model with ln(odds) = b0+ b1X1 +...+bp Xp, doesn't provide a better fit than the model without the independent variables resulting in, ln(odds) = b0.
Table 2. Sociodemographic variables among SIBO, and control groups
|
Variables |
SIBO negative (N=100) |
SIBO positive (N=100) |
P Value |
|
Age, yr |
56.5±14.1 |
57.3±15.0 |
0.240 |
|
Male, No (%) |
72 (72) |
75 (75) |
0.502 |
|
Body mass index, kg/m2 |
24.2±4.3 |
25.4±4.1 |
= 0.045 |
|
Smoking, No (%) Constipation, No (%). Methane +ve, No (%) Hydrogen +ve, No (%) |
32 (32%) 17 (17%) 12 (12%) 14 (14%) |
35 (35%) 45 (45%) 46 (46%) 27(27%) |
= 0.653 < 0.0001 < 0.0001 = 0.023 |
There are significant differences between SIBO cases, and control regarding constipation, methane test positivity, hydrogen test positivity and body mass index. It is noted that the difference is higher and more significant in both constipation and methane positivity. The logistic regression model doesn't provide a better fit than the model without the independent variables.
Table 3. Comparison of laboratory tests among diseased groups and control groups
|
|
Category |
Urea Breath test Positive. |
Odds ratio, (95% CI) |
P value |
H.P stool antigen test Positive. |
Odds ratio, (95% CI) |
P value |
Methane Breath test Positive |
Odds ratio, (95% CI) |
P value |
||
|
I 1 |
IBS, NO, 125 (%) |
87(69.6%) |
23.149, (10.5717 to 50.6904) |
< 0.0001 |
53(42.4%) |
(3.785 to 18.932) |
P < 0.0001 |
48(38.4%) |
4.571(2.264 to 9.230 |
P < 0.0001 |
||
|
1 |
SIBO, NO=100 (%) |
70(70.0%) |
|
< 0.0001 |
56(56.0%) |
14.636 (6.425 to 33.343) |
P < 0.0001 |
46(46.0%) |
6.247(3.041 to 12.834 |
P < 0.0001 |
||
|
1 |
Control, NO, 100 (%) |
9(9.0%) |
|
|
8(8.0%) |
|
|
12(12.0%) |
|
|
Comparing laboratory tests (UBT, HP stool Ag, MBT) among groups, there are highly significant differences between diseased (IBS, SIBO) and control groups.
Table 4. Comparison of UBT, and HP Stool antigen test among diseased groups
|
Category |
Urea Breath test Positive |
Odds ratio, (95% CI) |
P value |
H.P Stool antigen test Positive |
Odds ratio, (95% CI) |
P value |
|
IBS, No= 125 |
87(69.6%) |
0.981(0.553 to 1.740) |
0.948 |
53(42.4%) |
0.578(0.340 to 0.983) |
0.0432 |
|
SIBO, No= 100 |
70(70.0%) |
|
|
56(56.0%) |
|
|
Although there is no significant difference in UBT test results between the diseased groups, there is significant difference in stool antigen test positivity between SIBO, and IBS, indicating that SIBO cases have more HP stool positivity than IBS cases.
Table 5. Comparison of methane and hydrogen breath tests between SIBO, and control group
|
Category |
Methane Breath Test |
Odds ratio, (95% CI) |
P value |
Hydrogen Breath Test |
Odds ratio, (95% CI) |
P value |
|
SIBO, No (%) |
46 (46) |
6.247 (3.041 to 12.834 |
< 0.0001 |
27(27) |
2.272 (1.109 to 4.653) |
= 0.025 |
|
Control, No (%) |
12 (12) |
|
|
14(14) |
|
|
The production of methane in SIBO cases is higher and more significant than hydrogen production.
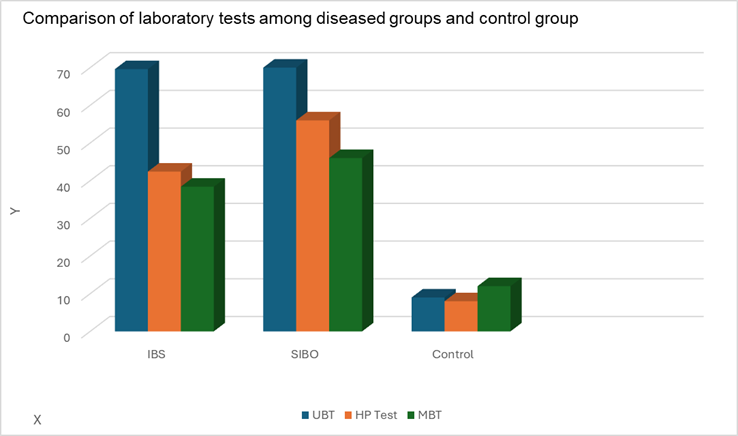
Fig 2. Shows comparative values of lab results in case and control groups.
IBS = Irritable Bowel Syndrome.
SIBO = Small Intestinal Bacterial Overgrowth.
UBT = Urea Breath Test.
HP Test = Helicobacter pylori Stool Antigen test.
MBT = Methane Breath Test
[1]. Kashyap P, Moayyedi P, Quigley EMM, Simren M, Vanner S. Critical appraisal of the SIBO hypothesis and breath testing: A clinical practice update endorsed by the European society of neurogastroenterology and motility (ESNM) and the American neurogastroenterology and motility society (ANMS). Neurogastroenterology & Motility. 2024; 36: e14817. doi:10.1111/nmo.14817
[2]. Wang C, Yin Y, Wang L, Guo X, Liu L, Qi X. Association between Helicobacter pylori infection and irritable bowel syndrome: a systematic review and meta-analysis. Postgrad Med J. 2023 May 19;99(1169):166-175. doi: 10.1136/postgradmedj-2021-141127. PMID: 37222050.
[3]. Wang Z, Liu Y, Peng Y, Peng L. Helicobacter pylori Infection-A Risk Factor for Irritable Bowel Syndrome? An Updated Systematic Review and Meta-Analysis. Medicina (Kaunas). 2022 Aug 2;58(8):1035. doi: 10.3390/medicina58081035. PMID: 36013502; PMCID: PMC9413972.
[4]. Abdelrazak MA. Walid FE, M Abdelrahman and Mohamed Abdeltawab M. Interrelation between Helicobacter Pylori Infection, Infantile Colic, and irritable bowel syndrome in Pediatric Patients. Journal of Medical and Biological Science Research (2015). Vol. 1 (7), ISSN: 2449-1810 Research Paper http://pearlresearchjournals.org/journals/jmbsr/index.html. https://api.semanticscholar.org/CorpusID:116157663
[5]. Xiong F, Xiong M, Ma Z, Huang S, Li A, Liu S. Lack of Association Found between Helicobacter pylori Infection and Diarrhea-Predominant Irritable Bowel Syndrome: A Multicenter Retrospective Study. Gastroenterol Res Pract. 2016; 2016:3059201. doi: 10.1155/2016/3059201. Epub 2016 Jul 17. PMID: 27493660; PMCID: PMC4967462.
[6]. Zhang J, Wang C, Han F, Liu D, Han Y. No evidence for a causal link between Helicobacter pylori infection and irritable bowel syndrome: a Mendelian randomization study. Front Microbiol. 2024 Feb 7; 14:1268492. doi: 10.3389/fmicb.2023.1268492. PMID: 38384720; PMCID: PMC10879563.
[7]. Tack J, Bisschops R, Sarnelli G. Pathophysiology and treatment of functional dyspepsia. Gastroenterology. 2004; 127(4): 1239–55. Doi: 10.1053/j.gastro.2004.05.030 [PubMed] [CrossRef] [Google Scholar].
[8]. Saffouri, G.B., Shields-Cutler, R.R., Chen, J. et al. Small intestinal microbial dysbiosis underlies symptoms associated with functional gastrointestinal disorders
Crossref DOI link: https://doi.org/10.1038/s41467-019-09964-7. Published Online: 2019-05-01
Update policy: https://doi.org/10.1007/springer_crossmark_policy.
[9]. Pesce M, Cargiolli M, Cassarano S, Polese B, De Conno B, Aurino L, Mancino N, Sarnelli G. Diet and functional dyspepsia: Clinical correlates and therapeutic perspectives. World J Gastroenterol. 2020 Feb 7;26(5):456-465. doi: 10.3748/wjg. v26.i5.456. PMID: 32089623; PMCID: PMC7015717.
[10]. Ali A, AlHussaini KI. Helicobacter pylori: A Contemporary Perspective on Pathogenesis, Diagnosis and Treatment Strategies. Microorganisms. 2024 Jan 22;12(1):222. doi: 10.3390/microorganisms12010222. PMID: 38276207; PMCID: PMC10818838.
[11]. Usarov K., Ahmedov A., Abasiyanik M.F., Ku Khalif K.M.N. Forecasting of Infection Prevalence of Helicobacter pylori (H. pylori) Using Regression Analysis. IIUM Eng. J. 2022; 23:183–192. doi: 10.31436/iiumej. v23i2.2164. [DOI] [Google Scholar]
[12]. Baj J, Forma A, Sitarz M, Portincasa P, Garruti G, Krasowska D, Maciejewski R. Helicobacter pylori Virulence Factors-Mechanisms of Bacterial Pathogenicity in the Gastric Microenvironment. Cells. 2020 Dec 25;10(1):27. doi: 10.3390/cells10010027. PMID: 33375694; PMCID: PMC7824444.
[13]. Konieczna I, Zarnowiec P, Kwinkowski M, Kolesinska B, Fraczyk J, Kaminski Z, Kaca W. Bacterial urease and its role in long-lasting human diseases. Curr Protein Pept Sci. 2012 Dec;13(8):789-806. doi: 10.2174/138920312804871094. PMID: 23305365; PMCID: PMC3816311].
[14]. Kim, Y.S. (2024). H. pylori Virulence Factors: Genetic Polymorphism and Disease
Crossref DOI link: https://doi.org/10.1007/978-981-97-0013-4_6. Published Online: 2024-03-01
Published Print: 2023. Update policy: https://doi.org/10.1007/springer_crossmark_policy
[15]. Deenadayalan K G, Kumaravel K,.Virulence factors of uropathogens and their role in host pathogen interactions.The Cell Surface, Vol. 8, (2022),100075, ISSN 2468-2330,
https://doi.org/10.1016/j.tcsw.2022.100075.
(https://www.sciencedirect.com/science/article/pii/S2468233022000044).
[16]. Subramaniyan, Y., Khan, A., Fathima, F. et al. Differential expression of urease genes and ureolytic activity of uropathogenic Escherichia coli and Pseudomonas aeruginosa isolates in different nutritional conditions Crossref DOI link: https://doi.org/10.1007/s00203-023-03722-6
Published Online: (2023)-11-16. Published Print: 2023-12. Update policy : https://doi.org/10.1007/springer_crossmark_policy.
[17]. Cheng K, Lee C, Garniene R, Cabral H, Weber HC. Epidemiology of Irritable Bowel Syndrome in a Large Academic Safety-Net Hospital. Journal of Clinical Medicine. 2024; 13(5):1314. https://doi.org/10.3390/jcm13051314.
[18]. Yang W, Yang X, Cai X, Zhou Z, Yao H, Song X, Zhao T, Xiong P. The Prevalence of Irritable Bowel Syndrome Among Chinese University Students: A Systematic Review and Meta-Analysis. Front Public Health. 2022; 10:864721. [PMC free article] [PubMed] [Google Scholar]
[19]. Huang KY, Wang FY, Lv M, Ma XX, Tang XD, Lv L. Irritable bowel syndrome: Epidemiology, overlap disorders, pathophysiology and treatment. World J Gastroenterol. 2023 Jul 14;29(26):4120-4135. doi: 10.3748/wjg. v29.i26.4120. PMID: 37475846; PMCID: PMC10354571.
[20]. Black, C.J., Ford, A.C. Global burden of irritable bowel syndrome: trends, predictions and risk factors. Nat Rev Gastroenterol Hepatol 17, 473–486 (2020). https://doi.org/10.1038/s41575-020-0286-8].
[21]. AGA Clinical Practice Update on Small Intestinal Bacterial Overgrowth: Expert Review Quigley, Eamonn M.M. et al. Gastroenterology, (October 2020), Vol. 159, Issue 4, 1526 – 1532.
[22]. Bushyhead D, Quigley EMM. Small Intestinal Bacterial Overgrowth-Pathophysiology and Its Implications for Definition and Management. Gastroenterology. 2022; 163:593–607. [PubMed] [Google Scholar].
[23]. Ghoshal UC, Sachdeva S, Ghoshal U, Misra A, Puri AS, Pratap N, Shah A, Rahman MM, Gwee KA, Tan VPY, Ahmed T, Lee YY, Ramakrishna BS, Talukdar R, Rana SV, Sinha SK, Chen M, Kim N, Holtmann G. Asian-Pacific consensus on small intestinal bacterial overgrowth in gastrointestinal disorders: An initiative of the Indian Neurogastroenterology and Motility Association. Indian J Gastroenterol. 2022; 41:483–507. [PMC free article] [PubMed] [Google Scholar]
[24]. Skrzydło-Radomańska B, Cukrowska B. How to Recognize and Treat Small Intestinal Bacterial Overgrowth? J Clin Med. 2022;11 [PMC free article] [PubMed] [Google Scholar].
[25]. Sharabi, E., Rezaie, A. Small Intestinal Bacterial Overgrowth. Curr Infect Dis Rep 26, 227–233 (2024). https://doi.org/10.1007/s11908-024-00847-7.
[26]. Ghoshal UC, Shukla R, Ghoshal U. Small Intestinal Bacterial Overgrowth and Irritable Bowel Syndrome: A Bridge between Functional Organic Dichotomy. Gut Liver. 2017 Mar 15;11(2):196-208. doi: 10.5009/gnl16126. PMID: 28274108; PMCID: PMC5347643.
[27]. Roszkowska P, Klimczak E, Ostrycharz E, Rączka A, et al. Small Intestinal Bacterial Overgrowth (SIBO) and Twelve Groups of Related Diseases-Current State of Knowledge. Biomedicines. 2024 May 7;12(5):1030. doi: 10.3390/biomedicines12051030. PMID: 38790992; PMCID: PMC11117733.
[28]. Bohm M., Shin A., Teagarden S., Xu H., Gupta A., Siwiec R., Nelson D., Wo J.M. Risk Factors Associated with Upper Aerodigestive Tract or Coliform Bacterial Overgrowth of the Small Intestine in Symptomatic Patients. J. Clin. Gastroenterol. 2020; 54:150–157. doi: 10.1097/MCG.0000000000001150. [DOI] [PMC free article] [PubMed] [Google Scholar].
[29]. Fish EM, Shumway KR, Burns B. Physiology, Small Bowel. [Updated 2024 Jan 31]. In: Stat Pearls [Internet]. Treasure Island (FL): Stat Pearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532263/.
[30]. Broekaert IJ, Borrelli O, Dolinsek J, Martin-de-Carpi J, Mas E, Miele E, Pienar C, Ribes-Koninckx C, Thomassen R, Thomson M, Tzivinikos C, Benninga M. An ESPGHAN Position Paper on the Use of Breath Testing in Paediatric Gastroenterology. J Pediatr Gastroenterol Nutr. 2022; 74:123–137. [PubMed] [Google Scholar]
[31]. Baker JR, Chey WD, Watts L, Armstrong M, Collins K, Lee AA, Dupati A, Menees S, Saad RJ, Harer K, Hasler WL. How the North American Consensus Protocol Affects the Performance of Glucose Breath Testing for Bacterial Overgrowth Versus a Traditional Method. Am J Gastroenterol. 2021; 116:780–787. [PubMed] [Google Scholar].
[32]. Madigan KE, Bundy R, Weinberg RB. Distinctive Clinical Correlates of Small Intestinal Bacterial Overgrowth with Methanogens. Clin Gastroenterol Hepatol. 2022; 20:1598–1605.e2. [PubMed] [Google Scholar].
[33]. Efremova I, Maslennikov R, Poluektova E, Vasilieva E, Zharikov Y, Suslov A, Letyagina Y, Kozlov E, Levshina A, Ivashkin V. Epidemiology of small intestinal bacterial overgrowth. World J Gastroenterol. 2023 Jun 14;29(22):3400-3421. doi: 10.3748/wjg. v29.i22.3400. PMID: 37389240; PMCID: PMC10303511].
[34]. Duller S, Moissl-Eichinger C. Archaea in the Human Microbiome and Potential Effects on Human Infectious Disease. Emerging Infectious Diseases. 2024;30(8):1505-1513. doi:10.3201/eid3008.240181.
[35]. James G. Volmer1, Harley McRae, Mark Morrison. The evolving role of methanogenic archaea in mammalian microbiomes. In Front. Microbiol. (2023) Sec. Biology of Archaea
Vol. 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1268451.
[36]. Gandhi A, Shah A, Jones MP, Koloski N, Talley NJ, Morrison M, Holtmann G. Methane positive small intestinal bacterial overgrowth in inflammatory bowel disease and irritable bowel syndrome: A systematic review and meta-analysis. Gut Microbes. 2021 Jan-Dec;13(1):1933313. doi: 10.1080/19490976.2021.1933313. PMID: 34190027; PMCID: PMC8253120].
[37]. Jahng J, Jung IS, Choi EJ, Conklin JL, Park H. The effects of methane and hydrogen gases produced by enteric bacteria on ileal motility and colonic transit time. Neurogastroenterol Motil. 2012 Feb;24(2):185-90, e92. doi: 10.1111/j.1365-2982.2011.01819. x. E pub 2011 Nov 20. PMID: 22097886].
[38]. Wang T, van Dijk L, Rijnaarts I, Hermes GDA, et al,. Methanogen Levels Are Significantly Associated with Fecal Microbiota Composition and Alpha Diversity in Healthy Adults and Irritable Bowel Syndrome Patients. Microbiol Spectr. 2022 Dec 21;10(6): e0165322. doi: 10.1128/spectrum.01653-22. E pub 2022 Nov 2. PMID: 36321894; PMCID: PMC9769613.
[39]. Guindo CO, Davoust B, Drancourt M, Grine G. Diversity of Methanogens in Animals' Gut. Microorganisms. 2020 Dec 23;9(1):13. doi: 10.3390/microorganisms9010013. PMID: 33374535; PMCID: PMC7822204.
[40]. Van de Pol J.A.A., van Best N., Mbakwa C.A., Thijs C., Savelkoul P.H., Arts I.C.W., Hornef M.W., Mommers M., Penders J. Gut colonization by methanogenic archaea is associated with organic dairy consumption in children. Front. Microbiol. 2017; 8:35. doi: 10.3389/fmicb.2017.00355. [DOI] [PMC free article] [PubMed] [Google Scholar].
[41]. Erasme Mutuyemungu, Mukti Singh, Sean Liu, Devin J. Rose. Intestinal gas production by the gut microbiota: A review, Journal of Functional Foods. Volume 100, (2023),105367, ISSN 1756-4646, https://doi.org/10.1016/j.jff.2022.105367.
(https://www.sciencedirect.com/science/article/pii/S1756464622004376)
[42]. Kalantar-Zadeh, K., Berean, K.J., Burgell, R.E. et al. Intestinal gases: influence on gut disorders and the role of dietary manipulations. Nat Rev Gastroenterol Hepatol 16, 733–747 (2019). https://doi.org/10.1038/s41575-019-0193-z.
[43]. Mann E.R., Lam Y.K. & Uhlig, H.H. Short-chain fatty acids: linking diet, the microbiome and immunity. Nat Rev Immunol 24, 577–595 (2024). https://doi.org/10.1038/s41577-024-01014-8.
[44]. N.W. Smith, P.R. Shorten, E.H. Altermann, N.C. Roy, W.C. McNabb
Hydrogen cross-feeders of the human gastrointestinal tract
Gut Microbes, 10 (3) (2019), pp. 270-288, 10.1080/19490976.2018.1546522
[45]. Arturo Tozzi, Raffaele Minella. Dynamics and metabolic effects of intestinal gases in healthy humans, Biochem., Vol. 221, (2024), Pages 81-90, ISSN 0300-9084,
https://doi.org/10.1016/j.biochi.2024.02.001.
(https://www.sciencedirect.com/science/article/pii/S0300908424000373).
[46]. Tansel A, Levinthal DJ. Understanding Our Tests: Hydrogen-Methane Breath Testing to Diagnose Small Intestinal Bacterial Overgrowth. Clin Transl Gastroenterol. 2023 Apr 1;14(4): e00567. doi: 10.14309/ctg.0000000000000567. PMID: 36744854; PMCID: PMC10132719.
[47]. Takakura W, Pimentel M, Rao S, et al. A single fasting exhaled methane level correlates with fecal methanogen load, clinical symptoms and accurately detects intestinal methanogen overgrowth. Am J Gastroenterol 2022;117(3):470–7. [PubMed] [Google Scholar].
[48]. Madigan KE, Bundy R, Weinberg RB. Distinctive clinical correlates of small intestinal bacterial overgrowth with methanogens. Clin Gastroenterol Hepatol 2022;20(7):1598–605.e2. [PubMed] [Google Scholar]
[49]. Khan MZ, Lyu R, McMichael J, et al. Chronic intestinal pseudo-obstruction is associated with intestinal methanogen overgrowth. Dig Dis Sci 2022;67(10):4834–40. [PubMed] [Google Scholar]
[50]. Singh P, Duehren S, Katon J, Rangan V, Ballou S, Patel R, Iturrino J, Lembo A, Nee J. Breath Methane Does Not Correlate with Constipation Severity or Bloating in Patients with Constipation. J Clin Gastroenterol. 2020 Apr;54(4):365-369. doi: 10.1097/MCG.0000000000001239. PMID: 31306344.
[51]. Gandhi A, Shah A, Jones MP, et al. Methane positive small intestinal bacterial overgrowth in inflammatory bowel disease and irritable bowel syndrome: A systematic review and meta-analysis. Gut Microbes 2021;13(1):1933313. [PMC free article] [PubMed] [Google Scholar].
[52]. Talamantes, S., Steiner, F., Spencer, S. et al. Intestinal Methanogen Overgrowth (IMO) Is Associated with Delayed Small Bowel and Colonic Transit Time (TT) on the Wireless Motility Capsule (WMC). Crossref DOI link: https://doi.org/10.1007/s10620-024-08563-x. Published Online: 2024-07-27. Published Print: 2024-09
Update policy: https://doi.org/10.1007/springer_crossmark_policy
[53]. Lacy BE, Patel NK. Rome Criteria and a Diagnostic Approach to Irritable Bowel Syndrome. J Clin Med. 2017 Oct 26;6(11):99. doi: 10.3390/jcm6110099. PMID: 29072609; PMCID: PMC5704116].
[54]. Rezaie A, Buresi M, Lembo A, et al. Hydrogen and methane‐based breath testing in gastrointestinal disorders: the North American consensus. Am J Gastroenterol. 2017; 112:775–784. https://doi.org/10.1038/ajg.2017.46
[55]. Gurusamy, Saravana Ruban; Shah, Ayesha MBBS, Talley, Nicholas J, et al, Small Intestinal Bacterial Overgrowth in Functional Dyspepsia: A Systematic Review and Meta-Analysis. The American Journal of Gastroenterology 116(5): p 935-942, May 2021. | DOI: 10.14309/ajg.0000000000001197.
[56]. Andrew C. Dukowicz, MD, Brian E. Lacy, PhD, MD, and Gary M. Levine, MD. Small Intestinal Bacterial Overgrowth: A Comprehensive Review. Gastroenterology & Hepatology Volume 3, Issue 2 February 2007.
[57]. Gahyun Baek, Bruce E. Logan, A comprehensive analysis of key factors influencing methane production from CO2 using microbial methanogenesis cells, Water Research, Vol. 245, (2023), 120657, ISSN 0043-1354, https://doi.org/10.1016/j.watres.2023.120657.
(https://www.sciencedirect.com/science/article/pii/S0043135423010977).
[58]. Gahyun Baek, Bruce E. Logan, A comprehensive analysis of key factors influencing methane production from CO2 using microbial methanogenesis cells, Water Research, Volume 245, 2023, 120657, ISSN 0043-1354, https://doi.org/10.1016/j.watres.2023.120657.
[59]. Yuan Jing, Jiayin Tian, Xin Cai, Rui Lin, Evolution and mechanism of impedance in PEMFC induced by cathode ammonia contamination, Fuel, Vol. 363, (2024), 130971, ISSN 0016-2361, https://doi.org/10.1016/j.fuel.2024.130971. (https://www.sciencedirect.com/science/article/pii/S0016236124001170)
(https://www.sciencedirect.com/science/article/pii/S0043135423010977).
[60]. Decong Zheng, Daping Li, Jingting Wang, Yifeng Zhang, Recent advances in anodic anaerobic ammonia oxidation in microbial electrolysis cells, Current Opinion in Electrochemistry, Vol. 45, (2024), 101470, ISSN 2451-9103, https://doi.org/10.1016/j.coelec.2024.101470.
(https://www.sciencedirect.com/science/article/pii/S2451910324000310).
[61]. H.T.T. Dinh, et al. Bioelectrical methane production with an ammonium oxidative reaction under the No organic substance condition. Microb Environ, 36 (2021). Google Scholar.
[62]. Kalantar-Zadeh, K.J. Berean, R.E. Burgell, J.G. Muir, P.R. Gibson
Intestinal gases: Influence on gut disorders and the role of dietary manipulations
Nature Reviews Gastroenterology and Hepatology, 16 (12) (2019), pp. 733-747, 10.1038/s41575-019-0193-z.
[63] Basseri RJ, Basseri B, Pimentel M, Chong K, Youdim A, Low K, Hwang L, Soffer E, Chang C, Mathur R (2012) Intestinal methane production in obese individuals is associated with a higher body mass index. Gastroenterol Hepatol 8:22–28.
[64]. Laverdure R, Mezouari A, Carson MA, Basiliko N, Gagnon J. A role for methanogens and methane in the regulation of GLP-1. Endocrinol Diabetes Metab. 2017 Dec 1;1(1): e00006. doi: 10.1002/edm2.6. PMID: 30815543; PMCID: PMC6353219].
[65]. Ye ZH, Ning K, Ander BP, Sun XJ. Therapeutic effect of methane and its mechanism in disease treatment. J Zhejiang Univ Sci B. 2020 Aug.;21(8):593-602. doi: 10.1631/jzus. B1900629. PMID: 32748575; PMCID: PMC7445089.
[66]. Poles, M.Z., Juhász, L. & Boros, M. Methane and Inflammation - A Review (Fight Fire with Fire). ICMx 7, 68 (2019). https://doi.org/10.1186/s40635-019-0278-6.
[67]. Yu Song and Yuan Liu and Baozhen Qi and Xiaotong Cui et al. Association of Small Intestinal Bacterial Overgrowth with Heart Failure and Its Prediction for Short‐Term Outcomes
Journal of the American Heart Association. Vol. 10, 7, pages e015292, (2021), Crossref DOI link: https://doi.org/10.1161/JAHA.119.015292. Published Print: 2021-04-06. Update policy: https://doi.org/10.1161/crossmarkpolicy
[68]. Fialho A, Fialho A, Schenone A, Thota P, McCullough A, Shen B. Association between small intestinal bacterial overgrowth and deep vein thrombosis. Gastroenterol Rep. 2016; 4:299–303. https://doi.org/10.1093/gastro/gow004. Google Scholar
[69]. Niu XL, Liu L, Song ZX, Li Q, Wang ZH, Zhang JL, Li HH. Prevalence of small intestinal bacterial overgrowth in Chinese patients with Parkinson's disease. J Neural Transm (Vienna). 2016; 123:1381–1386. https://doi.org/10.1007/s00702-016-1612-8. PubMed, Google Scholar.
[70]. Malik A, Morya RK, Bhadada SK, Rana S. Type 1 diabetes mellitus: complex interplay of oxidative stress, cytokines, gastrointestinal motility and small intestinal bacterial overgrowth. Eur J Clin Invest. 2018;48: e13021. https://doi.org/10.1111/eci.13021. PubMed, Google Scholar
[71]. Ponziani FR, Pompili M, Di Stasio E, Zocco MA, Gasbarrini A, Flore R. Subclinical atherosclerosis is linked to small intestinal bacterial overgrowth vitamin K2‐dependent mechanisms. World J Gastroenterol. 2017; 23:1241–1249. Crossref, PubMed, Google Scholar.
[72]. He Z, Ding R, Wu F, Wu Z, Liang C. Excess alcohol consumption: a potential mechanism behind the association between small intestinal bacterial overgrowth and coronary artery disease. Dig Dis Sci. 2018; 63:3516–3517. https://doi.org/10.1007/s10620-018-5279-x.
[73]. Singh R, Zogg H, Wei L, et al. Gut microbial dysbiosis in the pathogenesis of gastrointestinal dysmotility and metabolic disorders. J Neurogastroenterol Motil. 2021; 27:19–34. doi: 10.5056/jnm20149. [DOI] [PMC free article] [PubMed] [Google Scholar].
[74]. Kountouras J, Papaefthymiou A, Polyzos SA,et al,. Impact of Helicobacter pylori-related Microbial Dysbiosis in the Pathogenesis of Metabolic Syndrome and Gastrointestinal Dysmotility Disorders. J Neurogastroenterol Motil. 2021 Oct 30;27(4):653-654. doi: 10.5056/jnm21059. PMID: 34642287; PMCID: PMC8521481.
[75]. Ciselle Meier, Kharis Burns, Catherine Manolikos, Daniel Fatovich, Damon A. Bell,
Hyperammonemia: review of the pathophysiology, etiology and investigation, Pathology,
Vol. 56, Issue 6, (2024), Pages 763-772, ISSN 0031-3025, https://doi.org/10.1016/j.pathol.2024.06.002.
(https://www.sciencedirect.com/science/article/pii/S0031302524001776).
[76]. Padappayil RP, Borger J. Ammonia Toxicity. [Updated 2023 Mar 11]. In: Stat Pearls [Internet]. Treasure Island (FL): Stat Pearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK546677/
[77]. Vacca, Irene. The microbiota maintains oxygen balance in the gut. Crossref DOI link.
: https://doi.org/10.1038/nrmicro.2017.112. Published Online: 2017-09-04
Published Print: 2017-10. Update policy: https://doi.org/10.1007/springer_crossmark_policy
[78]. Noor A, Khetarpal S. Anaerobic Infections. [Updated 2023 Apr 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482349/.
[79]. Morais BP, Magalhães CP, Martins G, Pereira MA, Cavaleiro AJ. Effect of micro-aeration on syntrophic and methanogenic activity in anaerobic sludge. Appl Microbiol Biotechnol. 2024 Feb 2;108(1):192. doi: 10.1007/s00253-023-12969-4. PMID: 38305902; PMCID: PMC10837232.
[80]. Nwabunwanne Lilian NWOKOLO, Matthew Chekwube ENEBE. Methane production and oxidation—A review on the pmoA and mcrA genes abundance for understanding the functional potentials of the agricultural soil, Pedosphere, 2024, ISSN 1002-0160,
https://doi.org/10.1016/j.pedsph.2024.05.006.
(https://www.sciencedirect.com/science/article/pii/S1002016024000304)
[81]. Diederich P, Geisberger T, Yan Y, Seitz C, Ruf A, Huber C, Hertkorn N, Schmitt-Kopplin P. Formation, stabilization and fate of acetaldehyde and higher aldehydes in an autonomously changing prebiotic system emerging from acetylene. Commun Chem. 2023 Feb 22;6(1):38. doi: 10.1038/s42004-023-00833-5. PMID: 36813975; PMCID: PMC9947100].
[82]. Acetylene as Fast Food. Implications for the development of life on anoxic primordial earth and in the outer solar system. Astrobiology 8, 45–58 (2008).
[83]. Julius Z.H. von Martels, Mehdi Sadaghian Sadabad, Arno R. Bourgonje, Tjasso Blokzijl, Gerard Dijkstra, Klaas Nico Faber, Hermie J.M. Harmsen, The role of gut microbiota in health and disease: In vitro modeling of host-microbe interactions at the aerobe-anaerobe interphase of the human gut, Anaerobe, Vol. 44, 2017, Pages 3-12, ISSN 1075-9964, https://doi.org/10.1016/j.anaerobe.2017.01.001.
(https://www.sciencedirect.com/science/article/pii/S107599641730001X).
[84]. Yin, Q., Wu, G. (2024). A Holistic Metabolic Pathway of Anaerobic Digestion Integrating Substrate Degradation, Electron Transfer, Energy Conservation, and Information Flow. In: Wu, G. (eds) Anaerobic Digestion. Green Energy and Technology. Springer, Cham. https://doi.org/10.1007/978-3-031-69378-6_2.
[85]. Lee, J., Lee, B.T., Kwon, M.S. et al. Metabolic modeling of microorganisms involved in anaerobic digestion. Biotechno. l Bioprocess E 29, 613–624 (2024). https://doi.org/10.1007/s12257-024-00128-z. Crossref DOI link: https://doi.org/10.1007/s12257-024-00128-z. Published Online: 2024-07-01. Published Print: 2024-08. Update policy: https://doi.org/10.1007/springer_crossmark_policy.
[86]. Thorgersen, Michael P. Schut, Gerrit J. Poole, Farris L. II, Haja, Dominik K. Putumbaka, Saisuki Mycroft, Harriet I. de Vries, Willem J. Adams, Michael W. “W.O obligately aerobic human gut microbe expresses an oxygen resistant tungsten-containing oxidoreductase for detoxifying gut aldehydes. Crossref DOI link: https://doi.org/10.3389/fmicb.2022.965625 Published Online: (2022). Update policy: https://doi.org/10.3389/crossmark-policy.
[87]. Schut, G. J., Thorgersen, M. P., Poole, F. L., Haja, D. K., Putumbaka, S., and Adams, M. W. (2021). Tungsten enzymes play a role in detoxifying food and antimicrobial aldehydes in the human gut microbiome. Proc. Natl. Acad. Sci. U. S. A. 118:8118. doi: 10.1073/pnas.2109008118
PubMed Abstract | Cross Ref Full Text | Google Scholar.
[88]. Zheng, Z., Zong, Y., Ma, Y. et al. Glucagon-like peptide-1 receptor: mechanisms and advances in therapy. Sig Transduct Target Ther 9, 234 (2024). https://doi.org/10.1038/s41392-024-01931-z.
[89]. Gribble, F.M., Reimann, F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat Rev Endocrinol 15, 226–237 (2019). https://doi.org/10.1038/s41574-019-0168-8.