Clinical Case Reports and Clinical Study
OPEN ACCESS | Volume 12 - Issue 5 - 2025
ISSN No: 2766-8614 | Journal DOI: 10.61148/2766-8614/JCCRCS
Priya Hays
Hays Documentation Specialists, LLC, San Mateo, CA, USA.
*Corresponding author: Priya Hays, Hays Documentation Specialists, LLC, San Mateo, CA, USA.
Received: July 09, 2024
Accepted: July 15, 2024
Published: July 18, 2024
Citation: Priya Hays. (2024) “Clinical Decision Making in Advanced and Metastatic Breast Cancer Cases between PARP Inhibitors and CDK4/6 Inhibitors: A Review.”, Clinical Case Reports and Clinical Study, 11(2); DOI: 10.61148/2766-8614/JCCRCS/183
Copyright: © 2024. Priya Hays. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background: PARP inhibitors and CDK 4/6 inhibitors have been studied extensively as model targeted agents for advanced and metastatic breast cancer with hormone receptor positive/HER2 negative molecular subtype, the most common in the patient population.
Method: A PUBMED search was conducted using the keywords PARP inhibitors and Breast Cancer and CDK4/6 inhibitors AND Breast Cancer and PARP inhibitors AND Hormone Receptor Positive Tumors and PARP inhibitors AND Toxicity and BRCA1/2 mutation testing AND HR+/HER2- Advanced Breast Cancer. The studies selected for this review are based on patients having the molecular subtype of HR+/HER2-, and the clinical trials chosen for the decision workflow developed here include cohorts who received PARP inhibitor or CDK4/6 inhibitor therapies, and had either favorable or less than favorable outcomes.
Results: A number of factors, including molecular subtype, genomic characteristics, risk factors such as adverse events and potential for resistance, and evidence for desired clinical outcome must be considered in this decision making process, most impacted by the presence of the BRCA mutation and hormone receptor status and the prognostic and predictive potential of each agent. When faced with a patient with advanced breast cancer, the paper suggests that either PARP or CDK4/6 inhibitors could potentially be administered as monotherapy or in combination and proposes a clinical decision making algorithm based on a review of the literature that would enable clinicians to choose the most suitable agent based on the molecular and genomic characteristics of the patient and desired clinical outcome.
Breast cancer (BC) is a common malignancy with a high degree heterogeneity; the most common molecular subtype being hormone-receptor-positive (HR+)/human epidermal growth factor receptor type 2 (HER2)-negative constituting about 75% of BC. Recent data have confirmed that despite early-stage diagnosis, breast cancer has 25% relapse rate with a median survival for advanced breast cancer of 40.2 months. [1] Factors contributing to breast cancer development include strong family history, BRCA1 and breast cancer gene 2 (BRCA2) gene mutations, alcohol intake and increased age. Triple negative breast cancers, or TNBC, which do not express estrogen receptor, progesterone receptor or HER2 have the worst prognosis, and are most prevalent in African-American women, [2] and have recently come under study for PARP/CDK4/6 inhibitor combination studies.
The PARP inhibitors olaparib, talazoparib, and veliparib and CDK4/6 inhibitors abemaciclib, pablociclib, ribociclib, are considered among the mainstays for standard therapy for the treatment of advanced breast cancer, and both targeted agent classes are considered model precision oncology drugs and are FDA-approved [2,3 4-7]. PARP inhibitors are targeted agents for HER2- patients with somatic and germline BRCA1/2 mutations; while CDK4/6 agents are cell cycle inhibitors that serve as targeted agents for HER2- breast cancer patients and are also hormone receptor positive.
With the clinical data based on efficacy as demonstrated by clinical studies in mind, conceptually the management of breast cancer patients could be based on either class of agent for the molecular subtype HR+/HER2- patients. and the choice of which therapy to consider could be complex considering the prolific number of clinical trials, outcomes and reviews that have been performed to date evaluating efficacy, toxicity profiles and mechanisms of resistance for each targeted therapy regimen. This article describes the mechanisms of action for each class of agents, describes their development in the use of this patient population, summarizes the clinical data outcomes on both classes of inhibitors for HR+/HER2- advanced breast cancer patients (Table 1 and Table 5), and describes the adverse events associated with each, and mechanisms of resistance. Recent literature has presented how to overcome resistance and the use of biomarkers for predicting outcomes with treatment to determine prognosis.
In the concluding sections of paper, a novel clinical data algorithm is constructed in a flowchart with the clinical trial that provides evidence for their use in that setting. This algorithm can be described with the patient first being tested for the BRCA mutation and a positive result would presumptively lead to PARP inhibitor administration. A negative result would presumably predicate the use of CDK4/6 inhibitors. Each therapy combination is highlighted by the relevant clinical trial that provides evidence of clinical efficacy, as shown in Table 1 and Table 5. In light of this algorithm, in similar patient groups, CDK4/6i have more robust outcomes and should be considered standard of care for patients. For example, in the PALOMA-1 trial, PFS was 20 months for palbociclib with aromatase inhbitor (HR 0.58). However, this algorithm is complicated by studies that have shown adverse event profiles and primary and acquired resistance for these agents. The novelty of this review has been revealed by studies that prescribe the application of BRCA1/2 testing in the clinical setting after patients who have relapse/recurrence after CDK4/6 administration as revealed by a real world observational study that showed that a certain percentage of patients who receive CDK4/6i are BRCA-positive.
2. Materials and Methods
A PUBMED search was conducted on October 15, 2023 using the keywords PARP inhibitors and Breast Cancer and CDK4/6 inhibitors AND Breast Cancer and PARP inhibitors AND Hormone Receptor Positive Tumors and PARP inhibitors AND Toxicity and BRCA1/2 mutation testing AND HR+/HER2- Advanced Breast Cancer.
3. Results
3.1 The Development of PARP Inhibitors for Locally Advanced and Metastatic Breast Cancer
Cancer growth is characterized by uncontrolled cellular proliferation “comprised of sustaining proliferative signalling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, and activating invasion and metastasis” [8]. However, underpinning these hallmarks is genome instability, which generates the genetic diversity that expedites and fosters multiple hallmark functions [9]. Genomic instability often results from altered DNA repair capabilities resulting in different cancer types. PARP, or poly (ADP-ribose) polymerase has been characterized as involved in DNA repair processes in particular. Specifically, PARP1 mediates base excision repair as a result of single-stranded DNA breaks.
PARP1 inhibition is necessary but insufficient to contribute to lethality since the DNA damage that results can be repaired by the alternative homologous recombination pathway, a DNA repair mechanism for double-stranded DNA breaks. Precancerous cells that are BRCA negative lead to genomic instability and cancer; however, these tumors are inherently sensitive to DNA damage response inhibitors such as PARPis. The role of breast-cancer associated genes or BRCA1 and BRCA2 mediate the homologous recombination pathway; thus loss of both PARP1 activity coupled with loss of one of the BRCA genes results in synthetic lethality and cell death, since mutated BRCA1 and BRCA2 genes in cells lose the homologous recombination function (Figure 1).
Early phase studies of the development of olaparib are a demonstration of translational medicine as its cytotoxicity and anti-tumor activity were first shown in cell lines and mice tumor models that were deficient in BRCA1/2 and responded to olaparib[3].
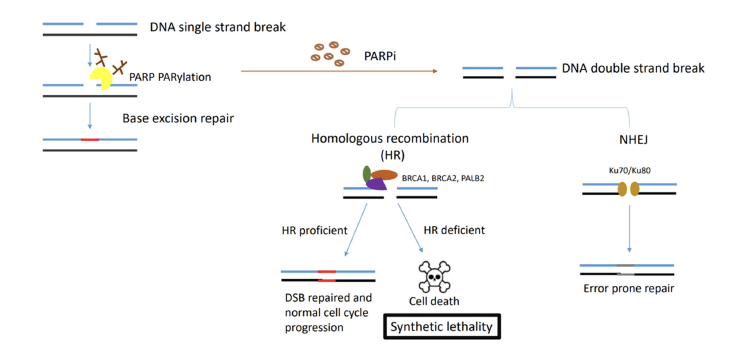
Figure 1 Synthetic Lethality of PARPs and BRCA1/2 mutations. PARP binds to ssDNA breaks while recruiting DNA damage repair proteins. In HR deficient cells associated with BRCA1/2 mutations and treated with PARP inhibitors, genome instability and cell death results since error prone repair pathways exist. (adapted from Wang et al 2023)
Of the five main PARP inhibitors evaluated veliparib, talazoparib and olaparib have shown clinical effectivity in advanced breast cancer.
Olaparib. In 2017, a phase III study OlympiAD was conducted in locally advanced or metastatic HER2 negative who harbored the gBRCA1/2 mutation. One cohort was randomized to receive olaparib monotherapy while the control group was treated with investigator chosen chemotherapy (capecitabine, gemcitabine, eribulin or vinorelbine). The trial met its primary endpoints of progression-free survival (PFS) in the olaparib arm vs physician’s choice: The olaparib arm demonstrated superior safety profile, being better tolerated than vs chemotherapy [8,10].
Menenez et al report that hormone-receptor positive disease was considered in the OlympiAD trial and provides more details on this study. Deleterious gBRCAm patients who received either two prior chemotherapy treatments or at least one endocrine therapy for HR+ positve disease were randomized to olaparib monotherapy or the standard-of-care chemotherapy. Patients were adminstered 300 mg olaparib and a 2:1 ratio of capecitabine, eribulin, or vinorelbine in 21-day cycles.[3]
Olaparib increased mPFS by nearly three months (7.0 months vs. 4.2 months; HR, 0.58; 95% confidence interval (CI), 0.43–0.80; p< 0.001). The ORR was 59.9% in the experimental group and 28.8% in the chemotherapy arm. The rate of grade 3 or higher AEs was 36.6% in the olaparib group vs 50.5% in the chemotherapy group. Treatment discontinuation as a result of toxicity was 4.9% vs 7.7% of patients, respectively, with no reported incidences of myelodysplastic syndrome (MDS), acute myeloid leukemia (AML), or other secondary malignancies [4, 11-13]. According to Menenez et al, “t]he FDA approved olaparib for the treatment of patients with gBRCAm and HER2-negative metastatic BC who have been treated with chemotherapy in the neoadjuvant, adjuvant, or metastatic setting, based on the findings of this study.” The study also reported that HR+ patients had progressed on prior endocrine therapy.[4,14]
Olaparib, Durvalumab, and Paclitaxel, Pusztai et al. in 2021 published the results of one arm of their phase II I-SPY2 adaptive platform study, which evaluated the combination therapy of durvalumab and olaparib with weekly paclitaxel for the neoadjuvant treatment of stage II/III, HER2-negative BC in 73 patients. The standard of care cohort was comprised of 299 patients. 14-28% of patient population in the HR+/HER2- arm was linked to a higher pCR rate in the durvalumab/olaparib/paclitaxel arm. In this arm, 12.3% of patients had grade 3 AEs constituting immune related symptoms, compared to 1.3% in the control arm. [15].
Veliparib. Veliparib in combinaton with carboplatin-paclitaxel was demonstrated in the BROCADE3 trial in 2020 as having clinical efficacy with prolonged PFS in gBRCAm locally advanced/metastatic breast cancer with HER2- subtype and HR+ subgroup. 509 patients were enrolled, and 266 or 5% were HR+ and 243(48%) were TNBC. PFS and OS results are shown in Table 1 derived from the research abstract by Ayoub et al, who also noted that “[a]dverse events (not related to progression) led to study drug discontinuation in 8.0% of HR+ [patients].”[16]
Talazaparib. According to Wang et al, “[t]he superiority of PARPi in gBRCA1/2 mutation-associated breast cancer was reaffirmed in another phase III study with talazoparib compared with a similar standard single agent chemotherapy” in the EMBRACA trial in 2018 (PFS was 8.6 vs 5.6 (HR 0.54; 95% CI 0.41 to 0.71; p < 0.001; ORR 62.6% vs 27.2%)). It was reported that HR+ patients were enrolled.[17,18].
Adverse Events and Resistance: Hematologic malignancies such as anemia and neutropenia, along with fatigue, were reported as the most common adverse events for PARP inhibitors. Anemia was more common when compared to neutropenia (39.2% versus 33.7%, RR 1.21, 95% CI 1.04 to 1.41, P = 0.01); (32.7% versus 52.0%, RR 0.67, 95% CI 0.60 to 0.76; P < 0.00001), respectively, and it was also reported by Taylor et al that “[f]atigue was non-significantly less common in patients receiving PARP inhibitors (32.0% versus 36.7%, RR 0.90, 95% CI 0.78 to 1.05, P = 0.18) and thrombocytopenia was also non-significantly less common in patients receiving PARP inhibitors (30.6% versus 35.8%, RR 0.98, 95% CI 0.84 to 1.15, P = 0.84)”. [19]
Causes of PARP resistance include (1) restoration of homologous recombination since HR-deficient cells develop reversion mutations that restore HR[20,21]; (2) prior exposure to chemotherapy that lead to upregulation of drug efflux pumps as a result of expression of the ABCB1 genes, also known as multidrug resistance genes; [22,23](3) mutations in the PARP1 molecule that decrease PARP trapping. Strategies to overcome PARP resistance have included adding immunotherapies such as immune checkpoint inhibitors since this enhances immunosurvellance. The TOPACIO study showed that when a PARPis are combined with the PD-1 inhibitor pembrolizumab[24-27]; and potential synergism takes place between the agents since both act on the “commonly dysregulated pathway in cancers: the phosphatidylinositol-4,5-biphosphate 3-kinase (PI3K)/AKT/mTOR pathway” [8,28,29]. Resistance brings challenges to PARP therapy and a number of strategies have been proposed to overcome it through therapy that would limit the time for the cancer cell to repair its damaged DNA.
Table 1 Summary of Clinical Trials for PARP inhibitors as Targeted Therapies
|
Trial |
Targeted Mutation |
Agent versus Comparator |
Dosing |
Primary Endpoints, |
Clinical Outcomes |
Adverse Effects |
|
|
gBRCAm |
Olaparib monotherapy vs control group treated with investigator chosen chemotherapy (capecitabine, gemcitabine, eribulin or vinorelbine). |
300 mg olaparib and a 2:1 ratio of capecitabine, eribulin, or vinorelbine in 21-day cycles |
PFS, ORR |
Increased mPFS by nearly three months (7.0 months vs. 4.2 months; HR, 0.58; 95% confidence interval (CI), 0.43–0.80; p< 0.001). ORR: 59.9% in experimental group and 28.8% in chemotherapy arm |
Rate of grade 3 or higher AEs 36.6% in the olaparib group vs 50.5% in the chemotherapy group. Treatment discontinuation as a result of toxicity was 4.9% vs 7.7% of patients, respectively, with no reported incidences of myelodysplastic syndrome (MDS), acute myeloid leukemia (AML), or other secondary malignancies |
|
BROCADE3 |
gBRCAm |
Veliparib in combinaton with carboplatin-paclitaxel(n=174 )vs placebo (n=92) |
Randomly assigned (2:1) to carboplatin 6 mg/mL per min intravenously) on day 1 and paclitaxel (80 mg/m2 intravenously) on days 1, 8, and 15 of 21-day cycles combined with either veliparib (120 mg orally twice daily, on days -2 to 5) or matching placebo. |
mPFS, mOS |
mPFS 13.0 (95% CI: 12.1, 16.6) mOS 32.4 (95% CI 26.5, 44.3); PFS HR .68 (0.48,0.97); OS HR 0.96(0.68, 13.6) |
Study drug discontinuation in 8.0%/3.3% of HR+ [patients |
|
EMBRACA |
gBRCA1/2 mutation associated breast cancer |
Talazaparib vs physician’s choice (capecitabine, eribulin, gemcitabine, vinorelbine) |
2:1 ratio, to receive talazoparib (1 mg once daily) or Standard single-agent therapy of the physician’s choice (capecitabine, eribulin, gemcitabine, or vinorelbine in continuous 21-day cycles) |
PFS, ORR |
Median progression-free survival: significantly longer in the Talazoparib group than in the standard-therapy group (8.6 months vs. 5.6 months; hazard ratio for disease progression or death, 0.54; 95% confidence interval [CI], 0.41 to 0.71; P<0.001). Interim median hazard ratio for death was 0.76 (95% CI, 0.55 to 1.06; P=0.11 [57% of projected events]). ORR:higher in talazoparib group than in the standard-therapy group (62.6% vs. 27.2%; odds ratio, 5.0; 95% CI, 2.9 to 8.8; P<0.001). |
Hematologic grade 3–4 adverse events (primarily anemia) occurred in 55% of the patients who received talazoparib and in 38% of the patients who received standard therapy; nonhematologic grade 3 adverse events occurred in 32% and 38% of the patients, respectively. |
|
I-SPY2 |
BRCA |
Combination therapy of durvalumab and olaparib weekly paclitaxel (DOP) |
Seventy-three participants were randomized to DOP and 299 to standard of care (paclitaxel) contro |
pCR |
DOP: increase in pathologic complete response (pCR) rates (27%-47%). MammaPrint ultra-high (MP2) cases benefited selectively from DOP (pCR 64% versus 22%), no benefit was seen in MP1 cancers (pCR 9% versus 10%). |
Overall, 12.3% of patients in the DOP arm experienced immune-related grade 3 adverse events versus 1.3% in control. |
3.2 The Development of CDK4/6 Inhibitors for Locally Advanced and Metastatic Breast Cancer
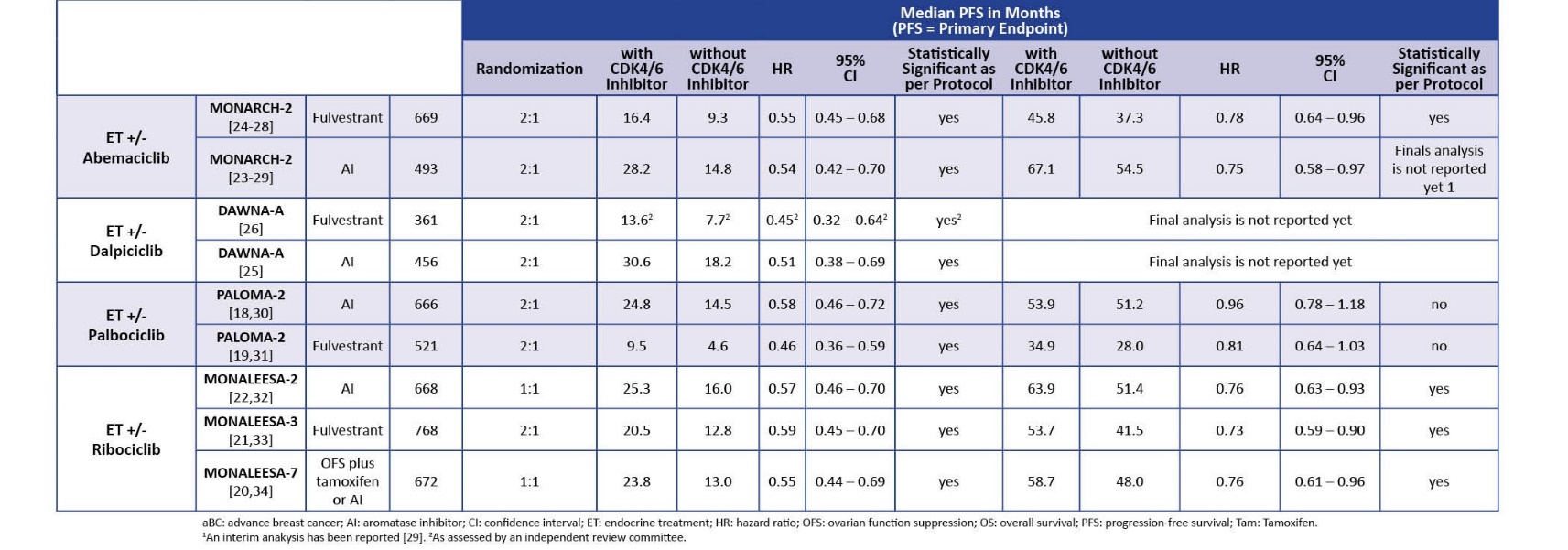
Table 2: Efficacy of CDK4/6 inhibitors in HR+/HER2- advanced breast cancer (adapted from Nabieva et al 2023)
CDK4/6 inhibitors came to play a significant role in the treatment of HR+ subtypes as a result of resistance to the standard treatments of anti-estrogen aromatase inhibitors, such as letrozole, and estrogen receptor antigonists, such as fulvestrant, that increase breast cancer mortality rates. CDK4/6 is involved in cell cycle maintenance by phosphorylating gatekeeper proteins such as the Rb protein that induce DNA synthesis.
“CDK4, and CDK6, together, with their cyclin-D regulatory subunits, promote the G1/S phase progression of the cell cycle.” (Figure 2) According to a review by Abdel et al, tumors are characterized by cell cycle dysregulation of a complex network consisting of cyclin dependent kinases that are responsible for cell cycle control, and among the CDKs, CDK4/6 lead to cell cycle progression. CDK4/6 associates with members of cyclin D, leads to cell cycle entry and progression into the G1 phase of the cell cycle. This complex phosphorylates the retinoblastina protein that leads to the release of the E2F transcription factor and overomes the G1 checkpoint and progression to S phase and into the S phase as a result of “activation of a cascade of downstream signaling promotes the activity of cyclin E/CDK2 complex, phosphorylation of other target proteins” [2].
Once CDK4/6 activity is regulated, an important step from the dormant state to cell cycle entry comes under control. This transition is further complicated by a network of other signaling cascades: RAS/MAPK and Pi3K/AKT/mTOR. However, the importance of this cascade becomes crucial to understanding the activity of CDK4/6 inhibitors in HR+ BC is that cyclin D1, implicated in this process as a partner of CDK4/6, is a signalling target of estrogen receptors and and overexprressed in HR+/HER2- BC, leading to “to continuous activation of the cyclin D1/CDK4/6 complex”[2]. Here, CDK4/6 inhibtors come into play since it leads to complete dephosphorylation of the RB protein and cell cycle progression haulting. This also explains the efficacy of CDK 4/6i. In combination with stimulation of tumor cells through estrogen dependency, ET causes a depletion of cyclin D1 and therefore a decrease in the construction of complexes with CDK4 and CDK6 and further explains how the assocation of CDK4/6i with ET inhibits BC. [2] Adding CDK4/6 inhibitors enhances endocrine therapy, increases efficacy and delays disease progression in the metastatic setting. “Current NCCN Guideline recommendations for metastatic HR+/HER2- breast cancer include the addition of CDK4/6Is with hormonal therapy (letrozole, fulvestrant) in postmenopausal and for premenopausal patients as a preferred first-line treatment.” Briefly, PALOMA-1 and PALOMA-2 have studied palbociclib + AI vs. placebo, MONALEESA-2 ribociclib vs. placebo and MONARCH-3 abemaciclib vs. placebo in patients not previously treated for MBC. PALOMA-3 for palbociclib, MONALEESA- 3 for ribociclib and MONARCH-2 for abemaciclib have showed the efficacy of CDK4/6i in association with fulvestrant in MBC patients with an endocrine-resistant disease. Taken together, all these studies have demonstrated a significant improvement in PFS and in some cases also in OS.
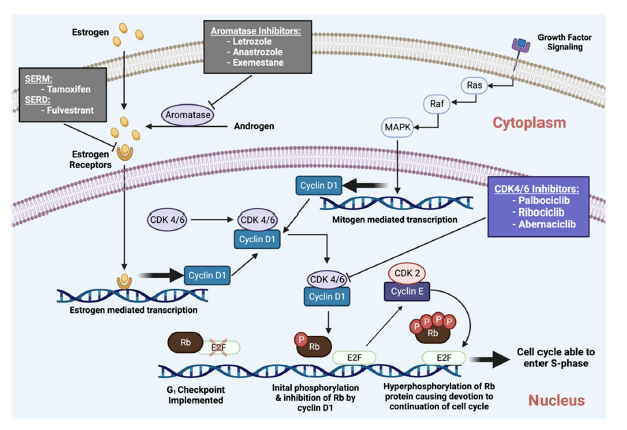
Figure 2 CDK4/6 inhibtors impact on the cell cycle and cellular death (adapted from Abdelmalak et al 2022)
The development of CDK4/6 inhibitors are also a prime example of translational research, since palbociclib was first evaluated and tested in human breast cancer lines, which led to its development as a result of its effect. Two phase III trials showed that the inhibitor significantly prolonged PFS, and the agent shortly became standard of care for HR+/HER2- patients who had advanced breast cancer. Similar results were shown for two subsquently developed CDK inhibitors, abemaciclib and ribociclib, that also demonstrated increased PFS. The results established these targeted agents for aBC since for 30 months patients were disease-free. [58]. Recent clinical studies have also shown that CDK4/6i are superior to chemotherapy in first and second line settings since according to a German breast cancer registry the rate of chemotherapy adminstration fell from 40% to 25% in first line settings for advanced breast cancer patients between 2015 to 2018 [58]. In 2021, the number fell to 85%, and this “rapid implementation” was further substantiated by the registry in a recent analysis that CDK4/6i monotherapy that had better prognosis than chemotherapy, which showed unfavorable prognosis. However, the study did acknowledge that this could be accounted for by a patient population that was high risk with worse prognosis. [39, 58]. The PEARL trial was conducted to confirm this superiority however statistical significance for pablociclib was not reached for PFS or OS compared to capcitabene.[58] Even further, palbociclib failed to improve invasive DFS in the phase III PALLAS and Penelope-B trials; however, this was in early treatment stages, establishing this agent in stage IV settings.
However the RIGHT choice trial did show an effect of ribociclib over chemotherapy analyzed in a pre and perimenopausal pateint population with visceral metastasis and aggressive diease. “In this patient population, it compared, as the first prospective trial, a ribociclib-based regimen to combinational chemotherapy in the first-line treatment setting. Ribociclib + ET could show a statistically significant PFS benefit of almost one year over chemotherapy (24.0 vs. 12.3 months; HR 0.54; 95% CI 0.36–0.79). On the basis of a better toxicity profile and quality of life (QoL), and at least similar or even better efficacy compared to chemotherapy, ET-based regimens in combination with CDK4/6i became the preferred treatment choice, even in patients with aggressive disease.” [58]
Despite the class effect of these agents in demonstrating positive PFS, the variation in OS outcomes was not easily explanable with the partly varied side effect profiles between them. In the PALOMA-2,3 trials, pablociclib failed to show any OS benefit (HR) of 0.81 and a 95% confidence interval (CI) of 0.64–1.03 in the PALOMA-3 trial, and a HR of 0.96 and a 95% CI of 0.78–1.18 in the PALOMA -2 trial. [ 58].
In contrast, abemaciclib demonstrated its efficacy in the MONARCH-2 trial with efficacy (HR 0.78; 95% CI 0.64–0.96) in combination with fulvestrant with prior chemotherapy treatment and a maximum of one prior ET for advanced breast cancer patients [28, 58]. Similarly, in the MONALEESA studies, ribociclib showed an improvement in mOS and OS as a first line agent independent or menopausal status or ET partner (AI or fulvestrant) [63]. The MONARCH-2 trial demonstrated a mOs of 63.9 months and OS prolongation of 12 months versus 51.4 months versus endocrine montherapy in aBC patients treated with ribociclib and letrozole as first-line therapy HR 0.76; 95% CI 0.63–0.93) [32] (Table 5). As Nabieva et al write, “It is of interest why these CDK4/6 inhibitors, despite being from the same drug family, lead to significantly different OS results. Potential reasons that are discussed are differences in the study designs and patient populations, but also in the substances’ pharmacology, affinity or in the binding to a specific side (more CDK4 than CDK6 and vice versa, for instance)” [58].
“There are also novel inhibitors of the CDK currently under development. Dalpiciclib, birociclib and lerociclib are considered new CDK4/6i that are being evaluated in patients with hormone receptor-positive, HER2-negative aBC within phase III studies in China. Trilaciclib, also a CDK4/6i, is being investigated in patients with triple-negative BC, with promising results. Dinaciclib, in contrast, inhibits the CDK1/2/5/9 and is also of interest for BC treatment. All these advancements show that CDKs role for the cell cycle are various and complex, and bear high potential for further development.”[58]
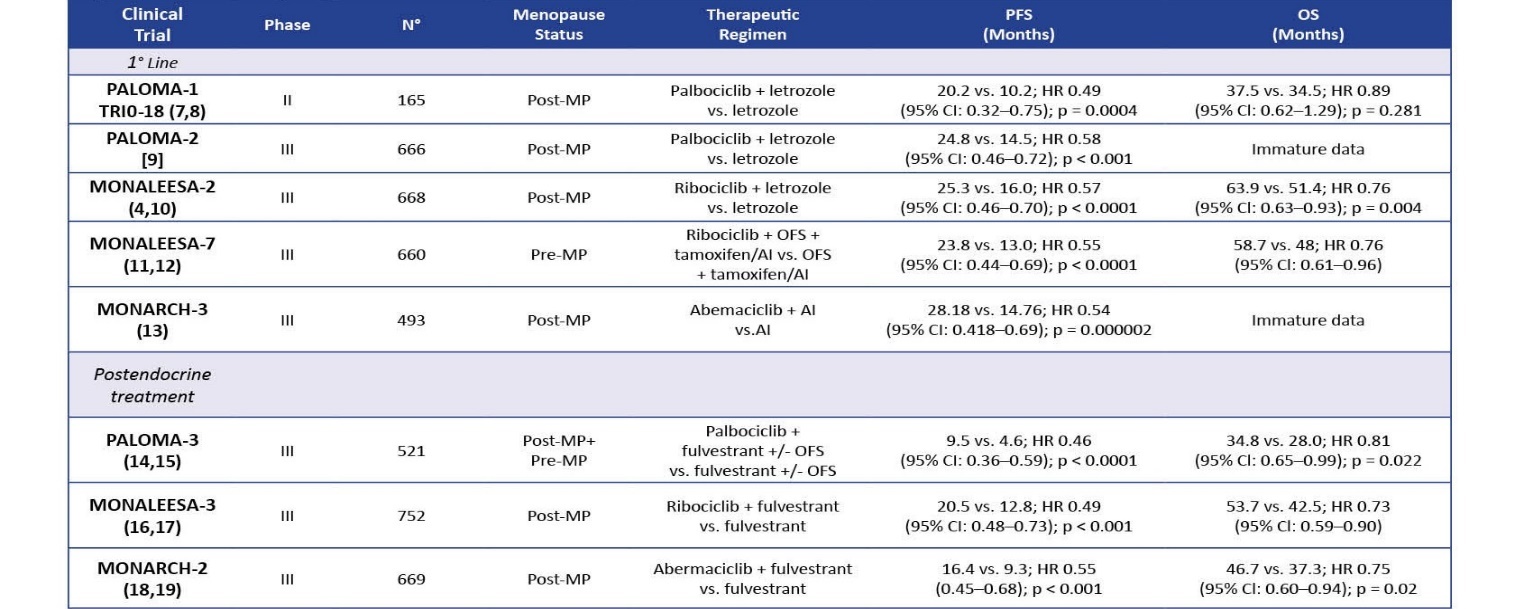
Table 3 Key Clinical Efficacy Data for CDK4/6 Inhibitors (adapted from Cogliati et al 2022)
Pablociclib was evaluated in a series of trials, the PALOMA-1 and PALOMA-2 in combination with letrozole in postmenopausal patients, which resulted in increased PFS in the experimental group versus patients taking letrozole alone. PFS: 20.2 vs. 10.2; HR 0.49 (95% CI: 0.32–0.75); p = 0.0004; OS: 37.5 vs. 34.5; HR 0.89 (95% CI: 0.62–1.29); p = 0.281. PALOMA-3 combined pablociclib with fulvestrant and also saw a change in PFS in patients 9.5 vs. 4.6; HR 0.46 (95% CI: 0.36–0.59); p < 0.0001 OS 34.8 vs. 28.0; HR 0.81 (95% CI: 0.65–0.99); p = 0.022. [1,2]
PALLAS, with endocrine therapy versus endocrine therapy alone, and it was concluded that “[p]albociclib is not recommended in the adjuvant setting of stage II/III ER+, HER2- breast cancer because the addition of Palbociclib to standard endocrine therapy (ET) did not improve outcomes.” Similarly PENELOPE-B which evaluated pablociclib with chemotherapy and did not meet its primary endpoint of invasive disease free survival [2,30].
Ribociclib, FDA approved in 2015 following pablociclib, is very similar in structure and function to pablociclib and most effective in combination with aromatase inhibitors including letrozole, but has a concerning side effect of cardiotoxicity, and must be monitored with EKGs. The MONALEESA set of trials evaluated whether ribociclib has any clincial effect over monotherapy with letrozole in ABC with HR+/HER2- subtype. MONALEESA-3 is a randomized phase III study with a cohort of ribociclib-fulvestrant, an ovarian function suppressor, in patients with advanced metastatic disease that prolonged PFS and OS (20.5 vs. 12.8 HR 0.59 (95% CI: 0.48–0.73); p < 0.001; 53.7 vs. 41.5; HR 0.73 (95% CI: 0.59–0.90)). [2]
Abemaciclib is also FDA-approved in 2017 for advanced breast cancer management in HR+/HER2- patients in a series of MONARCH trials that established clinical efficacy for abemaciclib monotherapy in both male and female patients who have relapsed after anti-hormonal agents and chemotherapy. MONARCH-1, a phase II research trial, evaluated abemaciclib monotherapy in this cohort with prior exposure to endocrine and chemotherapy. MONARCH-2, a phase III trial that randomized patients to a abemaciclib + fulvestrant combination versus placebo, showed higher PFS and OS rates for the combination therapy. A distinct feature of abemaciclib is that it can cross the blood-brain barrier and can reduce mortality in cases of CNS metastasis in breast cancer. PFS: 16.4 vs. 9.3; HR 0.55 (0.45–0.68); p < 0.001; OS: 46.7 vs. 37.3 HR 0.75 (95% CI: 0.60–0.94); p = 0.01.[1,3,4] “Administration of abemaciclib is provided continuously daily if well tolerated (a twice-daily regimen is permitted), which differs from the dosing schedule for other CDKIs.“[2]
Adverse Events and Resistance: The most common side effects include bone marrow suppression and hepatotoxicity and gastric toxicity and less severe ones as pancytopenia, particularly febrile neutropenia, which are monited by a differential CBC. [31-33] Ribociclib is noted for having cardiotoxic adverse effects that also must be monited with EKGs, since dose-prolongation QT intervals are associated with 600 mg doses “Toxic effects of ribociclib are similar to those of palbociclib, with the addition of cardiotoxic side effects with ribociclib. These effects are monitored with routine EKGs, as stated earlier. Dose-dependent prolongation of the QT interval is seen at a dose of 600 mg.” [2]. While mechanisms underlying endocrine therapy resistance have been identified such as “upregulation of ER cofactors (FOXA1 for example), cyclins (particularly D and E), CDK proteins (CDK2 and 6), pathways of mitogenic signaling (PI3K and RAS)”, current knowledge of CDK4/6 resistance and its underlying mechanisms remains unclear, with a recent review reporting that this has only been studied in vitro in cell line models. [1, 34-36]
Adverse events, though predictable, did not impact the widespread adoption of CDK4/6i. A meta-analysis or randomized trials on 3685 patients showed that the most common toxicity was hematological toxicity such as neutropenia and anemia along with fatigue and diarrhea. According to one review, neutropenia is considered a “class side effect of CDK4/6 inhibitors, and grade 3–4 neutropenia were very common in the PALOMA, MONALEESA, and MONARCH clinical trials, with an incidence of 65% in palbociclib, 58% with ribociclib and lowest with abemaciclib (22–27%).” Febrile neutropenia remained at a low 2%, much less than chemotherapy, that was managed by dose reduction.
There are distinguising features among the main three agents in terms of side effects. Diarrhea occurs more frequently with abemaciblib, the most potent inhbiitor; the MONARCH-2 trial showed a 13.4% incidence, managed with increased fluid intake. Among the three, ribociclib increases the risk of ventricular tachycardia leading to venticular fibrillation, since it causes prolongation of the QTc interval. “In randomized ribociclib trials, QTc interval prolongation experienced by patients was reversible and managed by dose interruption and reduction, without any clinical consequences. Treatment with ribociclib is recommended only in patients with QTc < 450 msec.” Thus, it is recommended not to be used in combination with agents that prolong QTc intervals. [63]
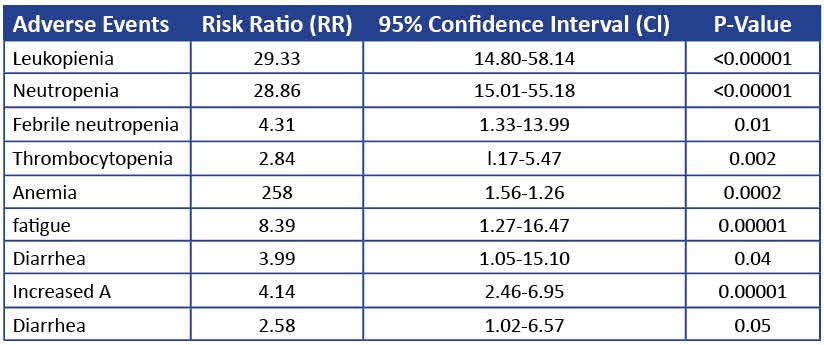
Table 4 Toxicities for CDK4/6i (adapted from Razeq 2022).
Resistance: CDK4/6 inhibitors have been shown to improve prognosis in this molecular subtype of HR+/HER2- patients; however, despite these robust outcomes primary and acquired resistance inevitably occur, leading to treatment discontinuation. This has significant implications for clinical decision making as noted by Krasniq et al, since they note that the “[t[he identification of differentially-expressed genes or genomic mutational signatures able to predict sensitivity or resistance to CDK4/6 inhibitors is critical for medical decision-making and for avoiding or counteracting primary or acquired resistance against CDK4/6 inhibitors.” [62]
“The first examples of acquired resistance were reported by Condorelli et al, where acquired RB1 mutations were detected in ER-positive breast cancer patients treated with palbociclib and fulvestrant or ribociclib and letrozole. To determine the function of Rb phosphorylation by cyclin D-CDK4/6, Topacio and colleagues sought to generate variants of Rb that could no longer interact with cyclin D-Cdk4,6 while preserving all the other interactions with other cyclin-Cdk complexes. They analyzed the docking interactions between Rb and cyclin D-CDK4/6 complexes and found that cyclin D-CDK4/6 targets the Rb family of proteins for phosphorylation, primarily by docking a C-terminal alpha-helix, which is not recognized by the other major cell-cycle cyclin-CDK complexes, including cyclin E-CDK2, cyclin A-CDK2, and cyclin B-CDK1. Their results showed that cyclin D-CDK4/6 phosphorylates and inhibits Rb via a C-terminal helix, and that this interaction is a major driver of cell proliferation.” [61]
The type of inhibitor associates with different resistance mechanism and leads to variable sensitivity of the agents targets, and onset of resistance is also distinguished by site of metastasis and prior therapies and outcomes such as DFS. Genomic aberrations to some extent are causative as well as are transcriptional changes. Predictive biomarkers play a role in determining adverse outcomes and “unveil targets” for precision medicine treatments to counteract resistance, primary or acquired. Several biomarkers have been identified as explained below, including mediators of the cell cycle.
“Inhibition of CDK4 and CDK6 leads to hypophosphorylation of RB1 and its family members. p130 and p107, resulting in binding and repression of transcription factor E2F, which is required for cell cycle progression. Cyclin E1 is the most prominent factor identified as upregulated in resistant BC . Overexpression of cyclin E1 leads to the activation and rewiring of CDK2, which enables the cell to bypass the cyclin D1-CDK4/6 blockade of RB1 and to enter a non-canonical S phase. High expression of CDK6 has also been reported to play an important role in the resistance mechanism. Increased expression of the cyclin-dependent kinase inhibitors 2D (CDKN2D, p19) and 2C (CDKN2C, p18), which belong to the INK4 family, has also been associated with reduced efficacy of CDK4/6 inhibitors plus suggesting that these tumors may have already lost their dependency on the CDK4/6 restriction point. In addition, upregulation of p16 (CDKN2A) at the protein level and an increase in the expression of E2F targets and other cell-cycle-related pathways, including Myc regulation, has been reported in patients with resistant BC, highlighting the critical role of these genes in the clinical efficacy of CDK4/6.”[64];
An overview of some of the mechanisms of resistance, such as loss of RB, caused by inactivation of the RB1 gene through mutation leading to constitutive activation of downstream proteins; E2F amplification; overexpression of the intrinsic tumor suppressor INK4 family, that can inhibit the formation of cyclin D‑CD K4/6 complex and inhibit the G1/S phase transition and thus decrease CDK4/6 binding to the complex; CDK amplification through epigenetics thus decreasing the blocking effect on cell cycle progression. Amplification of CDK4 leads to overactivation of proliferative pathways, but is an uncommon event; loss of ER/PR expression also can occur. Other cellular events implicated in resistance:
“Krasniqi et al. summarized in their study that some miRNAs (such as miR-326, miR- 29b-3p, miR-126, and miR3613-3p) are associated with sensitivity to CDK4/6 inhibitors, whereas others (such as miR-432-5p, miR-223, and miR-106b) appear to confer treatment resistance” [63]. Endocrine resistance and CDK4/6i sensitivity have also been studied as an association that would implicate clinical decision-making, and some resistance mechanisms are common to both types of therapies. The flowchart in Figure 4 shows that as a result of CDK4/6 resistance possible options are: switch to chemotherapy; combine with another targeted agent; or combine with inhibitors that can overcome resistance to mTOR and PI3K.
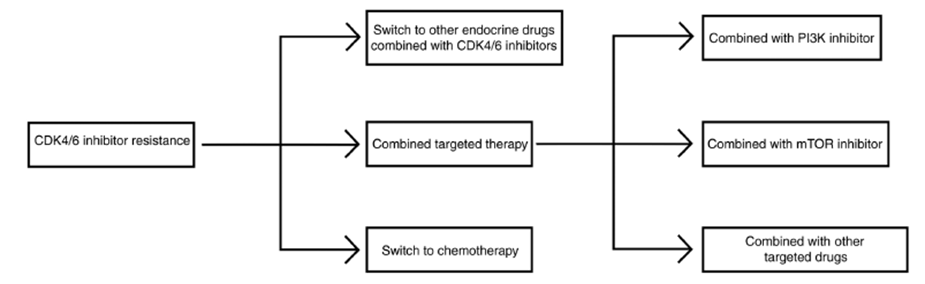
Figure 4 Workflow for CDK4/6 inhibitor administration after resistance (adapted from Huang et al 2022)
Liquid biospsy most utilized in the form of circulating tumor DNA has been analyzed in trials evaluating CDK4/6 and revealing potential biomarkers with prognostic and predictive potential. Most studies recommend to isolate samples in metastatic sites rather than the primary tumor to assess tumor heterogenetity and genomic status. For instance, in the PALOMA-3 trial loss of RB1 and KRAS mutation were associated with worse PFS in patients treated with palbociclib and fulvestrant. Similar data has been evinced for CCND1, PIK3CA, TP53, MYC, CCND1, CDK4, CDKN1, CDKN2, NF1, and ERBB2 alterations [45,49] that are also associated with CDK4/6i reistance. [58]. Mutations in Rb1 demonstrated preliminary evidence as a predictve biomarker since in the PALOMA-3 and MONALEESA trials ctDNA levels were associated with worse PFS, however the prevalence of this mutation was to low to be considered an association [39].
Similar results were shown for TP53 alterations, which were associated with progression but could not predict response. KRAS has shown to be a promising prognostic biomarker since one study showed that 106 HR+/HER2- subtype patients had a positive mutant KRAS ctDNA result showed progressive disease, while only one wild type KRAS patient had progressed[60].
3.3 Suggested Clinical Decision Making Algorithm for HR+/HER2- advanced or metastatic breast cancer patients
Since studies have established that HR+/HER2- molecular subtypes can be targeted by both PARP inhibitors and CDK4/6 inhibitors for advanced and metastatic breast cancer, this paper suggests a working hypothesis that based on the unique mechanisms and clinical outcomes each type of agent has against the tumor results in the design of clinical decision algorithm. This algorithm or flowchart, illustrated in Figure 3, shows that after determining an HR+/HER2- subtype, gBRCA1/2 testing may be performed to determine if PARP inhibitors are suitable if the test is positive. Four PARPi therapy regimens may be considered: an olaparib monotherapy, talazoparib monotherapy, or veliparib/carboplatin/paclitaxel combinaton or olaparib/durvalumab/paclitaxel combination. If the gBRCA1/2 test is negative, a CDK4/6 FDA-approved targeted therapy may be considered based on first-line or postendocrine status AND post- or pre-menopausal status. The algortihm displays the noted clinical trial and year in each case along with the pablociclib, ribocliclb or abemaciclib combination therapy, or in cases of CNS metastasis, abemaciclib monotherapy, since it has been reported that abemaciclib has the ability to cross the blood-brain barrier unlike the other targeted agents [2, 37, 42]
Two trials reported, the phase III SONIA and PALMIRA trials in 2023 performed an evaluation of CDK4/6 inhibitors in comparison with endocrine therapy alone that CDK 4/6 inhibitors. CDK4/6i did not improve PFS or OS and led to decrease in quality of life and financial toxicity. The phase III SONIA trial cohort included 1050 premenopausal and postmenopausal patients with HR+/HER2- patients that received a first-line aromatase inhibtor plus a CDK4/6 inhibitor [investigator’s choice] followed by fulvestrant; while, the phase II PALMIRA trial showed that pablociblib did not lead to improved PFS or OS in a similar patient population compared to second line endocrine therapy (fulvestrant or letrozole). PALMIRA reported tha “[m]edian overall survival for first-and second-line treatments were 45.9 months and 53.5 months,respectively (HR = 0.98; P = .83). There was no difference in quality of life according to Functional Assessment of Cancer Therapy–Breast scores”. [43,44]
This may indicate to administer CDK4/6 inhibitors in the second line setting after recurrence on endocrine therapy or to identify BRCA1/2 status in the “selection of therapy of patients already diagnosed with breast cancer, which indicate that [d]etermining BRCA1/2 mutation status in this breast cancer subgroup could potentially expand treatment options beyond the current standard of taxane and anthracycline-based chemotherapy” as reported in a 2018 review[45]. The 2020 real world, observational BREAKOUT study screened HR+/HER2- patients for gBRCA mutation status testing who experienced progression on endocrine therapy. Blood samples were tested for gBRCA mutation status and for somatic BRCA testing on archival tissue testing. Chemotherapy toxicity information was also collected. 341 patients were screened from 14 countries and testing and showed that gBRCAm prevalence was higher than suggested by traditional risk factors (5.8%), and also confirmed to be clinically relevant for therapy selection for patients already diagnosed with breast cancer.[45, 46] This would suggest that patients who have relapsed after receiving CDK4/6 inhibitors could undergo BRCA1/2 testing for potential PARP therapy, perhaps a novel recommendation. As Figure 4 shows, this avenue may be new in the literature when compared with the established avenues proposed. In this context, a review by Tung and Garber highlight that while testing for the BRCA positive is predictive in breast cancer risk assessment, studies have shown that identifying this mutational status would be clinically relevant in the selection of therapy for breast cancer patients who have already been diagnosed, which would implicate CDK4/6i in this setting.
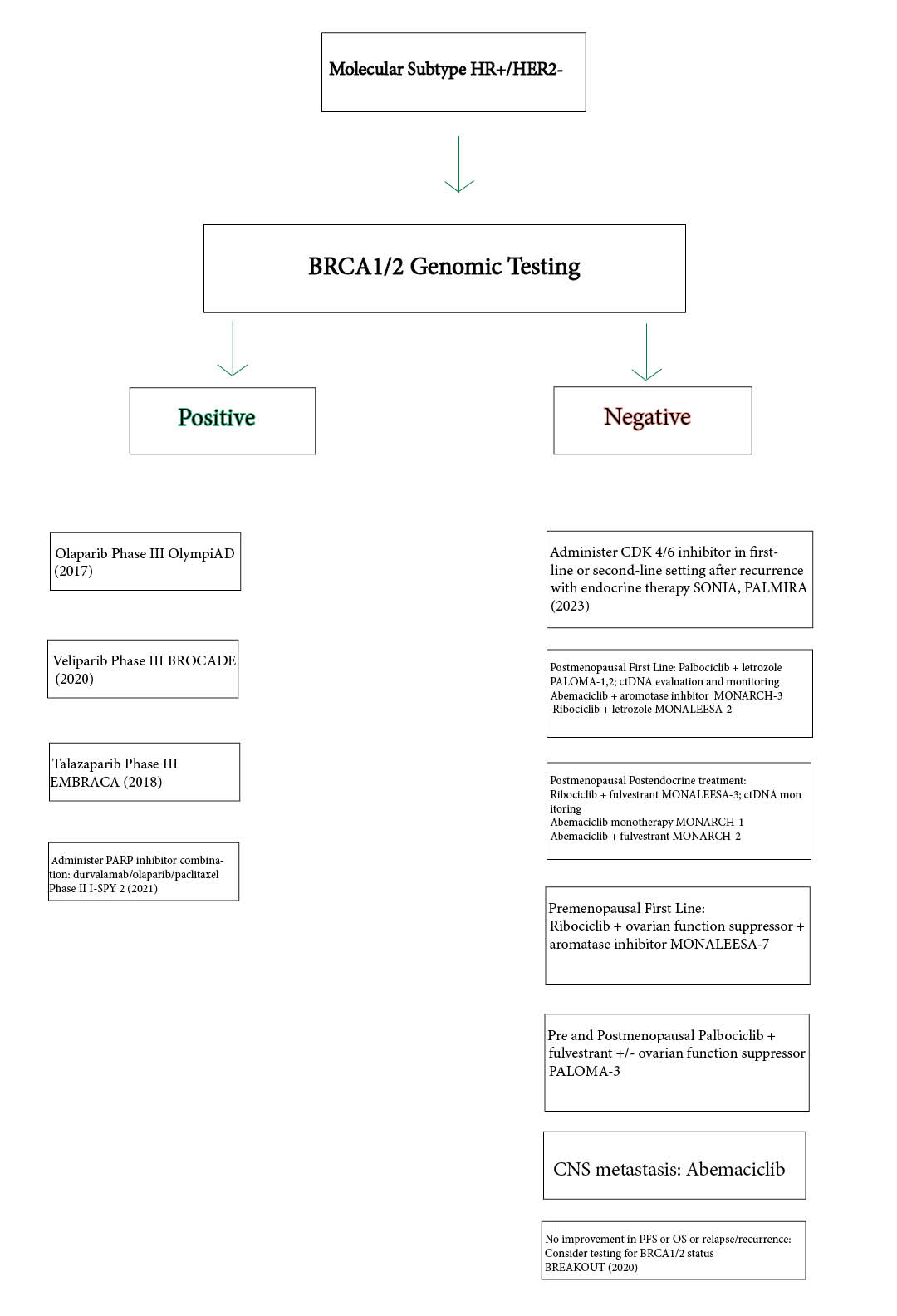
Figure 5 . Clinical Decision Making Algorithm for Locally Advanced or Metastatic Breast Cancer (see 3.3 for details)
Table 5 Summary of Clinical Trials for CDK4/6 inhibitors as Targeted Therapies
|
Trial |
Agent vs comparator |
Dosing |
Primary Endpoint |
Clinical Outcomes |
Adverse Effects |
|
PALOMA 2 |
Pablociclib in combination with letrozole in postmenopausal patients in the experimental group versus patients taking letrozole alone |
Postmenopausal women with ER+/HER2– ABC who had not received prior systemic therapy for advanced disease were randomized 2:1 to receive PAL (125 mg/d orally, 3/1 week schedule) plus LET (2.5 mg/d orally, continuously) or PBO+LET |
PFS
|
PFS: 20.2 vs. 10.2; HR 0.49 (95% CI: 0.32–0.75); p = 0.0004 |
The most common all-cause grade 3 or 4 adverse events in the palbociclib arm were neutropenia (92%) and leukopenia (29%); febrile neutropenia occurred in 4.1% of patients.
|
|
PALOMA-3 |
Pablociclib wiith fulvestrant vs fulvestrant alone; premenopausal and postmenopausal Asians taking palbociclib plus fulvestrant (n = 71) or placebo plus fulvestrant (n = 31). |
Patients were randomly assigned 2:1 to receive palbociclib plus fulvestrant or placebo plus fulvestrant. Patients received placebo or palbociclib 125 mg/d orally for 3 weeks followed by 1 week off; fulvestrant 500 mg was administered intramuscularly on days 1 and 15 of cycle 1 and then every 28 days (± 7 days) thereafter starting from day 1 of cycle 1.1 |
PFS |
PFS in patients 9.5 vs. 4.6; HR 0.46 (95% CI: 0.36–0.59); p < 0.0001 OS 34.8 vs. 28.0; HR 0.81 (95% CI: 0.65–0.99); p = 0.022 |
No new safety signals were observed.
|
|
PENELOPE-B |
Pablociclib with chemotherapy |
Patients were randomly assigned (1:1) to receive 13 cycles of palbociclib 125 mg once daily or placebo on days 1-21 in a 28-day cycle in addition to endocrine therapy (ET). |
Invasive disease-free survival |
After a median follow-up of 42.8 months (92% complete), 308 events were confirmed. Palbociclib did not improve iDFS versus placebo added to ET-stratified hazard ratio, 0.93 (95% repeated CI, 0.74 to 1.17) |
|
|
MONARCH-3 |
Double-blind, randomized phase III study of abemaciclib or placebo plus a nonsteroidal aromatase inhibitor in 493 postmenopausal women |
Patients received abemaciclib or placebo (150 mg twice daily continuous schedule) plus either 1 mg anastrozole or 2.5 mg letrozole, daily. |
Investigator-assessed progression-free survival. |
Median progression-free survival was significantly prolonged in the abemaciclib arm (hazard ratio, 0.54; 95% CI, 0.41 to 0.72; P = .000021; median: not reached in the abemaciclib arm, 14.7 months in the placebo arm). In patients with measurable disease, the objective response rate was 59% in the abemaciclib arm and 44% in the placebo arm ( P = .004). |
In the abemaciclib arm, diarrhea was the most frequent adverse effect (81.3%) but was mainly grade 1 (44.6%). Comparing abemaciclib and placebo, the most frequent grade 3 or 4 adverse events were neutropenia (21.1% v 1.2%), diarrhea (9.5% v 1.2%), and leukopenia (7.6% v 0.6%).
|
|
MONALEESA-2 |
Ribociclib vs letrozole, vs letrozole alone |
Randomly assigned the patients to receive either ribociclib (600 mg per day on a 3-weeks-on, 1-week-off schedule) plus letrozole (2.5 mg per day) or placebo plus letrozole. |
PFS |
Duration of progression-free survival was significantly longer in the ribociclib group than in the placebo group (hazard ratio, 0.56; 95% CI, 0.43 to 0.72; P=3.29×10−6 for superiority). The median duration of follow-up was 15.3 months. After 18 months, the progression-free survival rate was 63.0% (95% confidence interval [CI], 54.6 to 70.3) in the ribociclib group and 42.2% (95% CI, 34.8 to 49.5) in the placebo group |
|
|
MONALEESA-3 |
Ribociclib plus fulvestrant showed a significant overall survival benefit over placebo plus fulvestrant |
N/A |
OS |
The estimated overall survival at 42 months was 57.8% (95% confidence interval [CI], 52.0 to 63.2) in the ribociclib group and 45.9% (95% CI, 36.9 to 54.5) in the placebo group, for a 28% difference in the relative risk of death (hazard ratio, 0.72; 95% CI, 0.57 to 0.92; P=0.00455). The benefit was consistent across most subgroups |
Adverse events were generally more frequent in the ribociclib group, and the most common grade 3 or 4 adverse events were neutropenia (57.1% in the ribociclib group and 0.8% in the placebo group) and leukopenia (15.5% in the ribociclib group and 0% in the placebo group). Other key grade 3 or 4 adverse events of special interest were hepatobiliary toxic effects (13.7% and 5.8%, respectively) and prolonged QT interval (3.1% and 1.2%, respectively). Grade 3 or 4 interstitial lung disease was observed in 1 patient (0.2%) in the ribociclib group and no patients in the placebo group. |
|
SONIA |
Patients were randomized 1:1 to receive strategy A (first-line treatment with an NSAI + CDK4/6i, followed on progression by fulvestrant (F)) or strategy B (first-line treatment with an NSAI, followed on progression by F + CDK4/6i). Choice between one of the available CDK4/6i (abemaciclib, palbociclib, ribociclib) was a stratification factor and left to the discretion of the treating physician |
|
The primary endpoint is time from randomization to second objective disease progression, as assessed by local investigators, or death (PFS2) |
After a median follow-up of 37.3 months (data cut-off 1 December 2022), median PFS2 was 31.0 months in strategy A versus 26.8 months in strategy B (hazard ratio 0.87; 95% confidence interval, 0.74 to 1.03; P=0.10). |
The number of grade ≥3 adverse events was 2782 for strategy A and 1620 for strategy B |
|
PALMIRA |
Pablociblib vs second line aromatase inhibitor |
Patients were randomly assigned (2:1 ratio) to receive P plus second-line ET (letrozole or fulvestrant, based on prior ET) or second-line ET alone. |
OS |
[M]edian overall survival for first-and second-line treatments were 45.9 months and 53.5 months, respectively (HR = 0.98; P = .83 |
No difference in Quality of Life |
4. Discussion
This paper describes a suggested general algorithmic framework for treating advanced and metastatic breast cancer patients with HR+/HER2- molecular subtype with either PARP inhibitors or CDK4/6 inhibitors. Eligiblity guidelines such as ECOG status and clinical data evaluating outcomes such as RECIST scores are not considered in depth here but are reviewed elsewhere [18]. CDK4/6 inhibition seems to have undergone more rigorous study, and more data exists for the clinically sound stratification of patients when undergoing CDK4/6 therapy, as shown by the working hypothetical algorithm based on previous reviews and studies outlined here. There may be no study in the literature directly comparing CDK4/6 inhibitors versus PARP inhibitors on similar groups since PARP inhibitors are for patients who have undergone testing for the BRCA mutation; while patients who receive the major CDK4/6 inhibitors do not explicity undergo genetic testing and are stratified by pre-menopausal and post-menopausal status. This clinical algorithm suggested in this paper may be novel in the literature.
However, while CDK4/6 inhibition has become a standard of care (which also being clarified further), a review conducted by Akhade et al reported that the FDA approval of abemaciclib may have been only “marginally positive” and “premature” since it was based on a Ki-67 of more than 20%, being the first of its kind, and Ki-67 is a marker of cellular proliferation not predictive for clinical efficacy, concluding “[t]here are no studies that have shown that Ki-67 alone can be used as a predictor of benefit for CDK inhibitors [or any drug for advanced breast cancer.” [42]. The FDA, however, in 2023 removed the Ki67 testing requirement for high-risk patients [47]. Although CDKis have been shown to have the best results in conjunction with endocrine therapy, palbociclib and ribociclib in particular, synergize well in combination with phosphoinositide 3-kinase (PI3K) and mammalian target of rapamycin (mTOR) inhibitors. [2]
Additionally SONIA and PALMIRA in 2023 have reported underperformance by CDK4/6 inhibitors with second-line endocrine therapy as the comparator, and may suggest that selection (approximately 5-10%) of BC patients undergo germline mutation in BRCA1/2 genes, and suggesting the use of the olaparib and talazoparib. The GRADE (IAMO) panel evaluated PARP inhibitors for advanced HR+/HER2- patients and “judged the benefit/harm balance probably in favor of the intervention, given the favorable impact in terms of PFS, ORR, and QoL at an acceptable cost in terms of toxicity.” The recommendation was “conditional in favor of PARP inhbitors” over chemotherapy but endorsed CDK4/6 inhibitors as the preferred first line in HR+/HER2 patients with positive gBRCA+ status [48]. The ESO-ESMO panel recommendation for advanced breast cancer issued in 2020 reported favorably on the use of CDK4/6 inhbitors before the use of PARP inhibitor in ER+ gBRCA associated advanced BC patients.[49]
The results of the BREAKOUT trial show that the selection of (approximately 5-10%) of BC patients for testing of germline mutation in BRCA1/2 genes, recommending the use of PARP inhibitor therapy. The recommendation to undergo BRCA testing in the clinical setting post CDK4/6i administration and evaluation as suggested by this algorithm may be considered a novel strategy.
This may imply that there is more robust evidence for PARP inhibitors, however this is limited by the comparatively small data for them, and it was also shown that talazaoparib was shown to have no significant improvement in OS over chemotherapy in one analysis reported in 2020 [50]. Future studies may be directed towards evaluating patient populations that are of other molecular subtypes, such as triple negative breast cancer, and determining prognostic indicators for these treatment regimens as guided by the suggested algorithm and additional clinical studies for HR+/HER2- advanced BC evaluating PARP inhibitors.
Shu et al in 2022 reported on “A Real-World Disproportionality Analysis of Olaparib: Data Mining of the Public Version of FDA Adverse Event Reporting System”. Common adverse events were hematoloigic ones, such as anemia, thrombocytopenia, and gastrointestinal ones, such as decreased appetite and nausesa. Renal dysfunction was also reported and infectious diseases, as well. The median onset of AEs was 61 days. They conclude that the “Results of our study were consistent with clinical observations, and we also found potential new and unexpected AEs signals for olaparib, suggesting prospective clinical studies were needed to confirm these results and illustrate their relationship. Our results could provide valuable evidence for further safety studies of olaparib.” Zimmerman et al also reported on “characteristics, treatment patterns, and clinical outcomes of real-world US patients with gBRCAm HER2-negative LA/mBC treated with talazoparib monotherapy were collected via retrospective chart review and summarized using descriptive statistics. Overall, talazoparib clinical outcomes in this real-world population are consistent with findings from EMBRACA.” [65, 66]
Conclusion
As Stanciu et al write, “CDK4/6 inhibitors remain a landmark for the treatment of hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer, being the most significant advance in the last decade. Various preclinical and translational research efforts have begun to shed light on the genomic and molecular landscape of resistance to these agents. As [shown] above, it is important to understand the mechanism of action of CDK4/6 inhibitors in order to target specific signaling pathways and predictive biomarkers of response, taking into consideration that intrinsic and acquired resistance could limit the activity of these inhibitors. In addition, one of the greatest challenges is distinguishing between mechanisms causing resistance to CDK4/6 inhibition and endocrine resistance.” [67]
A renaissance likewise has occurred for PARP inhibitors and olaparib in particular has undergone extensive study and was later revealed in the OlympiAD trial to have significant OS results. Their use after CDK4/6i administration relapse/recurrence enabling the testing for the BRCA1/2 mutation in clinical settings, or after a breast cancer diagnosis is made and treatment administered is an option that may now be considered. Further studies by the oncology and precision medicine community may shed light on this proof-of-concept algorithm to address limitations such as side effects and resistance.
Author Contributions: P.H. wrote the manuscript in its entirety.
Funding: N/A
Institutional Review Board Statement: N/A
Informed Consent Statement: N/A
Data Availability Statement: N/A
Conflicts of Interest: P.H. has stock ownership in Eli Lilly.
Eli Lilly is the manufacturer of abemaciclib, one of the therapies discussed in this paper. This conflict did not influence the data presented or discussions pertaining to the clinical outcomes, side effects and resistance mechanisms pointed out. Specifically, the phase III trials SONIA and PALMIRA, both studies conducted in 2023 on abemaciclib, are discussed in detail. Both of these trials offered less favorable result and showed the limitations of using abemaciclb for this molecular subtype.