
Ahmed Khadraji*, Mohammed Bouhadi, Cherki Ghoulam
Unit of Plant Biotechnology and Symbiosis Agro-physiology, Faculty of Sciences and Techniques, PO. Box 549, Gueliz 40000 Marrakesh, Morocco.
*Corresponding Author: Ahmed Khadraji, Unit of Plant Biotechnology and Symbiosis Agro-physiology, Faculty of Sciences and Techniques, PO. Box 549, Gueliz 40000 Marrakesh, Morocco.
Received: May 04, 2021
Accepted: May 07, 2021
Published: May 17, 2021
Citation: A.Khadraji, M.Bouhadi and Cherki.G. (2021) “Effect of pH medium on the phosphorus solubilization at some isolates of rhizobium nodulating chickpea (Cicer arietinum L.)”, Journal of Agricultural Research Pesticides and Biofertilizers, 1(1); DOI:http;//doi.org/05.2021/1.1003.
Copyright: © 2021 Ahmed Khadraji. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This research aims to select strains of rhizobium nodulating chickpea (Cicer arietinum L.) with high production of available phosphorus collected from the root nodules of chickpea cultivated in 10 farms located in the south of Morocco. 68 isolates were obtained from the chickpea nodules, where only 12 strains were selected for their strong potential for nodulation. In order to evaluate strains tolerance, many abiotic stresses were assessed for the studied rhizobia such as temperature (4 and 40 °C), salinity (2.5, 3.5,4 and 5%) and pH levels tolerance (3, 3.5,11 and 12). Bacterial phosphate-solubilizing ability in NBRIP medium has demonstrated the ability of the studied strains to mobilize significant amounts of P from tricalcium phosphate (TCP), the isolates tested showed high P solubilization capacities like RhTmg 1, 5 and 10 with a solubilization diameter of P tricalcium greater than 1.8cm and which has also showed high tolerance to acidity. In pH 3 only the RhTmg 1, 5 and 10 isolates were able to grow. According to the obtained results, P solubilizing ability was directly correlated to the pH decrease in the medium
1. Introduction:
Chickpea (Cicer arietinum L.) is an important source of fiber, protein, and plays a key role in the sustainable development of agriculture because of its ability to fix atmospheric nitrogen in soils (El-Enany et al., 2013; Mantri et al., 2013). In Morocco, chickpeas are the second major food legume after beans. It is mainly cultivated in rainfed culture (Madrid et al., 2015). The total area of chickpeas cultivated in Morocco is estimated at around 60 200 ha with a yield of 718 kg ha-1 and a production of around 42 600 t (FAOSTAT, 2017). Despite this, the national production of the chickpea culture is low and does not meet the consumption of the population. This is mainly due to biotic and abiotic stress.
Morocco like other countries is affected by climate change which brings a negative impact more on plant production and therefore on chickpeas too. Thus, the culture is exposed to stressful conditions during the reproductive stage, resulting in yield losses (Houasli et al., 2020). Consequently, it is necessary to develop strains nodulating chickpea which resist the climate of the regions arid and semi-arid. This species could provide 144 Kg per ha per year of N to the soil in association with rhizobium strain (Unkovich and Pate, 2000). Several studies have reported the key role of stress tolerant rhizobia strains in the enhancement of legume biomass production through its direct effects on root and N2-fixing amelioration (Wang et al., 2016). In Morocco, the inoculation with bacteria strains is little known. It is used only in experimental basis (Khadraji et al., 2016). During the last decades, harvesting chickpea has been gradually extended from semi-arid to arid areas where environmental conditions have negative effects on the establishment of functional and efficient N2-fixing symbioses (Drevon and Sifi, 2000). Although non-native rhizobia strains could be used as inoculums in order to relive these problems, but these strains are generally less competitive and may result in less efficient N2-fixing under these drastic conditions. An efficient symbiosis depends first on both partners, the host legume and rhizobia strain as well as the environmental conditions. In response to drought stress, some chickpea genotypes of early plant stage were more droughts tolerant in comparison to others (Khadraji et al., 2016). This variability could be ameliorated in the presence of tolerant rhizobia strains in the growth stage. This may due to its positive effects on relative water content, photosynthesis rate and nutrient uptake under this condition (Mouradi et al.,2016).
Many researches have reported that phosphate solubilizing bacteria (PSB) could enhance crop yields via their high ability to solubilize the applied phosphates as well as those fixed in the soil (Zaidi et al.,2003). Indeed, beside its N2-fixing, several rhizobia strains have shown important activity of inorganic phosphorus solubilization to the plant (Ahemad et al., 2014). Generally, micro-organisms solubilizing inorganic P produce organic acids with low molecular weight such as carboxylic and ketogluconic acids (Richardson et al.,2001). This operation is also responsible for the reduction of the pH of the media (Rodriguez, 2007). In order to produce highly efficient N2 fixing chickpea-rhizobia symbioses that could be recommended in the arid and semi-arid regions of Morocco and grow even under stressful conditions, the objective of the present work was to study several characteristics of rhizobia strains nodulating chickpea (Cicer arietinum L.) and to select competitive native ones taking into account their tolerance to salinity, temperature, acidity as well as P solubilizing ability.
2. Material and methods:
2.1. Strains isolation:
The strains isolation has been collected from the root nodules of chickpea grown in 10 farms located in the Douar Timgret region of El Oualidia province of Sidi Bennour. It is a region with an agricultural vocation located in the geographical coordinates along 32 ° 36'06.8 "N 8 ° 55'35.0" W. The root nodules were detached, washed and then surface-sterilized by immersion in a sodium hypochlorite solution 6% during 1 min and then rinsed several times with sterile distilled water. Individual nodules were after that grounded in physiological water and an aliquot of 20 µL was used in Yeast Extract Mannitol (YEM) plates supplemented with Congo red. Thereafter, the plates were incubated for 48h at 28°C (Vincent, 1970).
2.2. Evaluation of the temperature, salinity and pH levels tolerance:
In order to evaluate temperature tolerance, the isolates were grown in YEM medium in different temperature (4 and 40 °C). Also, to evaluate salt tolerance of purified rhizobia strains, the isolates were grown in YEM medium with different percentage of NaCl (2.5, 3.5,4 and 5%) and in the control medium with a concentration of 1.7 mM of NaCl. Three replicates per isolate were performed. Bacterial growth was assessed after 48 h of incubation at 28°C.To study the effect of pH on bacterial growth, five YEM medium were prepared with different pH: 3,3.5,11 and 12.
2.3. Phosphate solubilization in solid medium with TCP as only source of P:
The rhizobia strains were cultivated on PVK medium (Pikovskaya, 1948). Solid, with Ca3PO4 as only source of P. This test allowed us to assess the capacity of the strains to dissolve P through their growth and the diameter of the halo surrounding the colonies. These clear areas are due to the production of organic acids in the environment surrounding the rhizobium colonies. After 5 days of incubation at 28°C, the phosphate solubilization of TCP has been demonstrated by the appearance of a clear halo around the colonies. The phosphate-solubilizing (PS) activity of the isolates was calculated in terms of phosphorus solubilization index (PSI) by using the formula PSI=A/B, where A is total of diameter of halo (mm) and B is the diameter of colony (mm). Isolates showing PSI≥2 were considered as phosphate-solubilizing bacteria (PSB) (Rahman et al.,2006)
3. Results and discussion:
A total of 68 rhizobia strains were isolated from collected nodules, where only 12 strains were passed the infectivity test with chickpea plants. These isolates appear after 24 to 48 hours on YEM agar medium.
3.1. Evaluation of the temperature, salinity and pH levels tolerance
The results (Table 1) showed that the ten isolates withstood the low temperature 4 ° C, on the other hand at the temperature 40 ° C only RhTmg 8 and 10 were able to resist. Also, for the salinity test note that a salinity of 2.5%, all the isolates arrived to grow except the strain RhTmg 4 and 7, but at salinity 5% of the medium observed that only the isolates RhTmg 1, 5, 8, and 10 were able to develop. Regarding the tolerance test to the variation of the pH medium, all of the isolates were able to grow in highly basic pHs, but in pH 3 only the RhTmg 1, 5 and 10 isolates were able to grow. Similar results were reported by Jida et al.,2012, who indicated that rhizobia strains can tolerate NaCl concentrations that exceed 342 mM and the limits of salt tolerance considerably vary from species to another and even between strains of the same species. Indeed, rhizobia strains nodulating species Melilotus indicus could tolerate pH levels between 4.5 and 9 (Arbi et al.,2015). In this sense, Indrasumunar et al. 2012, reported that Bradyrhizobium japonicum strains could tolerate a pH level of 3.8. The physiological and biochemical mechanisms of adaptation of rhizobia under acidic conditions are numerous. These mechanisms include, among others, the exclusion and expulsion of H+ protons polyamines accumulation (Kurchak et al.,2001). Isolated rhizobia nodulating pea that can tolerate pH values that exceed 11 (Rao et al., 2002).
The salt stress aects the growth and the rhizobia strains in the soil. The optimal concentration of NaCl for rhizobia is 1.5 %. Several strains are capable of growing in medium containing high concentrations of salt (> 3 %), in particular Sinorhizobium meliloti (Chen et al., 2000). Mesorhizobium ciceri is one of the most saline tolerant symbiotic bacteria and can form stable symbioses under salt stress (200mM) (Soussi et al., 2001).
The salt stress aects
the growth and the rhizobia strains in the soil. The optimal concentration of NaCl for rhizobia is 1.5 %. Several strains are capable of growing in medium containing high concentrations of salt (> 3 %), in particular Sinorhizobium meliloti (Chen et al., 2000). Mesorhizobium ciceri is one of the most saline
tolerant symbiotic bacteria and can form stable symbioses under salt stress (200mM) (Soussi et al., 2001).
The salt stress aects the growth and the rhizobia strains in the soil. The optimal concentration of NaCl for rhizobia is 1.5 %. Several strains are capable of growing in medium containing high concentrations of salt (> 3 %), in particular Sinorhizobium meliloti (Chen et al., 2000). Mesorhizobium ciceri is one of the most saline tolerant symbiotic bacteria and can form stable symbioses
under salt stress (200mM) (Soussi et al., 2001)
|
Strains |
Temperature (°C) |
PH |
Salinity (%) |
||||||||
|
4 |
40 |
3 |
3.5 |
11 |
12 |
2.5 |
3.5 |
4 |
5 |
||
|
RhTmg1 |
+ |
- |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
|
RhTmg2 |
+ |
- |
- |
- |
+ |
+ |
+ |
- |
- |
- |
|
|
RhTmg3 |
+ |
- |
- |
- |
+ |
+ |
+ |
+ |
+ |
- |
|
|
RhTmg4 |
+ |
- |
- |
- |
+ |
+ |
+ |
- |
- |
- |
|
|
RhTmg5 |
+ |
- |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
|
RhTmg6 |
+ |
- |
- |
+ |
+ |
+ |
+ |
+ |
+ |
- |
|
|
RhTmg7 |
+ |
- |
- |
- |
+ |
+ |
+ |
- |
- |
- |
|
|
RhTmg8 |
+ |
+ |
- |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
|
RhTmg9 |
+ |
- |
- |
+ |
+ |
+ |
+ |
+ |
+ |
- |
|
|
RhTmg10 |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
Table 1: Evaluation of the temperature, salinity and pH levels tolerance
3.1.5. Tricalcium phosphate (TCP) solubilization:
The results showed that there is a highly significant difference (P <0.001) in the P solubilization capacity between the different strains tested (Figure 1). The isolates tested showed high P solubilization capacities like RhTmg 1, 5 and 10 with a solubilization diameter of P tricalcium greater than 1.8cm, the other strains showed intermediate solubilization. The RhTmg 4 and 7 isolates did not show the ability to dissolve phosphorus.
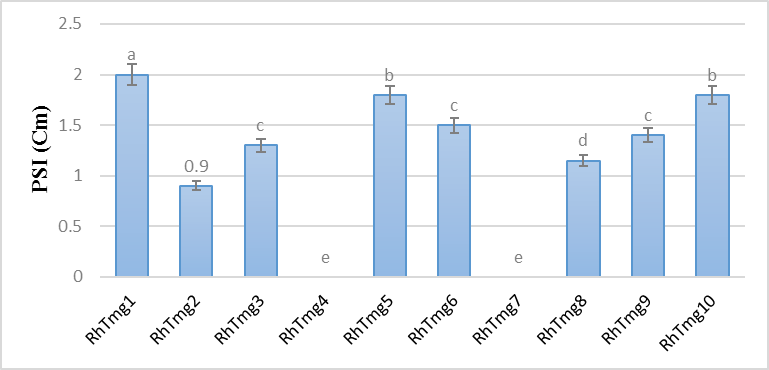
Figure.1: solubilization diameter of P tricalcium on solid medium.
Likewise, Whitelaw (2000), Jeon et al. (2003) and Zhao et al.(2005) who have shown that the solubilization of phosphorus is correlated with the decrease in the pH of the medium, which explains the capacity of these three strains (RhTmg 1, 5 et 10) to grow in a highly acidic medium since they already have the power to acidify the middle. Along the same lines, Pradhan and Sukla,2005 and Perez et al. (2007) have also shown that inorganic P is solubilized by organic acids secreted by bacteria, which chelate cations (Al, Fe, Ca) and lower the pH in the medium. Thus, the phosphorus solubilization capacity has a direct correlation with the pH of the medium (Fankem et al., 2006).
4. Conclusion:
The analysis of the physiological characteristics of the rhizobia isolates has highlighted the physiologic variations existed between all of the tested strains under different abiotic circumstances. The evaluation of major abiotic stress tolerance of selected strains has helped to distinct two rhizobia groups based on their stress tolerance abilities. The most tolerant group is formed by MRp6 and MRp8 strains which can tolerate higher NaCl concentrations (1711 mM). As well as their ability to grow in NBRIP agar and broth and the utilization of TCP as sole P source, this solubilization is strongly correlated with the decrease pH of the medium. The results showed that there was high consistence between this solubilizing power and growth speed for all of the studied strains especially those classified as more stress tolerant in comparison with the less tolerant ones.