Aditum Journal of Clinical and Biomedical Research
OPEN ACCESS | Volume 7 - Issue 1 - 2025
ISSN No: 2993-9968 | Journal DOI: 10.61148/2993-9968/AJCBR
Ogunbiyi, O. J1,2*., Apata, J. T3., Iyare, H. E1., Uchechukwu, C. A2. Owoh-Etete, U2. & Amusan, T. O4
1Department of Biochemistry, Faculty of Life Sciences, University of Benin, Benin City, Nigeria.
2Biology Unit, Faculty of Science, Air Force Institute of Technology, Kaduna, Nigeria.
3Department of Chemical Science, Dominion University Ibadan, City of Faith, Oyo State, Nigeria.
4Chemistry Department, Faculty of Science, Air Force Institute of Technology, Kaduna, Nigeria.
*Corresponding author: Ogunbiyi, O. J, 1Department of Biochemistry, Faculty of Life Sciences, University of Benin, Benin City, Nigeria.
Received: May 20, 2025
Accepted: June 15, 2025
Published: July 20, 2025
Citation: Ogunbiyi, O. J., Apata, J. T., Iyare, H. E., Uchechukwu, C. A. Owoh-Etete, U. & Amusan, T. O., (2025) “Comparative Haematotoxicity of Cadmium and Iron in Wistar Rats: Independent and Combined Exposure Effects” Aditum Journal of Clinical and Biomedical Research, 7(1); DOI: 10.61148/2993-9968/AJCBR/116
Copyright: ©2025. Ogunbiyi, O. J. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background: Cadmium (Cd) and iron (Fe) are environmental metals with known haematotoxic effects. While Cd is a well-established toxicant, Fe may exhibit dual roles as both essential and potentially toxic. This study investigates the haematological responses of adult male Wistar rats exposed to Cd and Fe, either individually or concomitantly, via tainted water and feed.
Methods: Rats were divided into groups and exposed to Cd and Fe either singly or in combination through drinking water and feed for a specified period. Haematological indices, including red and white blood cell (RBC and WBC) counts, haemoglobin (Hb), packed cell volume (PCV), thrombocytes, and differential leucocyte counts, were analysed. Erythrocyte indices such as mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), and mean corpuscular haemoglobin concentration (MCHC) were also evaluated.
Results: Exposure to Cd and Fe, particularly via feed, induced significant changes in several haematological parameters. Notably, a significant reduction in RBC, WBC, and thrombocyte counts was observed in rats exposed to Fe alone. Co-exposure to Cd and Fe led to further alterations in WBC and erythrocyte indices such as MCV and MCH. However, some parameters, such as PCV, neutrophils, lymphocytes, monocytes, eosinophils, and basophils, remained unaffected across treatment groups. Interestingly, Fe appeared to mitigate some of the haematotoxic effects induced by Cd in certain parameters.
Conclusion: The findings suggest that both Cd and Fe can independently and synergistically alter haematological indices in rats, with Fe showing potential protective as well as toxic effects. These outcomes highlight the complexity of metal-metal interactions and the need for further mechanistic studies on their combined toxicological impact.
Cadmium toxicity, Iron exposure, Haematological parameters, Tainted water, Tainted feed, RBC count
Cadmium (Cd) is a toxic heavy metal and a pervasive environmental contaminant that poses significant risks to human and animal health. Its widespread use in industrial processes and its persistence in the environment have led to increased exposure through contaminated food, water, and air (Satarug et al., 2017). However, human exposure through food and drinking water is greater than through air (Nordberg et al., 2015). Among its numerous toxic effects, Cd is known to impair hematological functions by disrupting erythropoiesis, inducing oxidative stress, and promoting red blood cell (RBC) hemolysis (Horiguchi et al., 2011; Yu et al., 2024). The hemotoxicity of Cd is largely attributed to its interference with essential trace elements, its ability to trigger lipid peroxidation in erythrocyte membranes, and its suppression of erythropoietin production, thereby leading to anemia (Sarkar et al., 1995; Horiguchi et al., 2011; Zhang et al., 2024).
Iron (Fe), a vital micronutrient, plays a central role in erythropoiesis, oxygen transport, and cellular respiration. It is a major component of hemoglobin and its availability directly influences red blood cell formation and function (Abbaspour et al., 2014; Donker et al., 2021). Importantly, Fe and Cd share similar transport pathways in the gastrointestinal tract, and Fe status can influence Cd absorption and toxicity. While iron deficiency enhances Cd uptake, adequate iron levels may confer a protective effect by reducing Cd bioavailability and oxidative damage (Włostowski et al., 2003; Zhai et al., 2015; Ezim et al., 2024).
Previous studies have demonstrated that iron supplementation can mitigate Cd-induced toxicity in various organs, including the liver and kidneys (Włostowski et al., 2003; Jamakala & Rani, 2015; Ezim et al., 2024). However, the specific modulatory effects of Fe on Cd-induced hemolysis and plasma hemolytic indices remain poorly understood. Understanding this interaction is critical, especially in populations at risk of concurrent exposure to both metals due to environmental or occupational hazards.
Therefore, this study aims to evaluate the hemotoxic effects of Cd and Fe administered singly and in combination in adult male rats, with a particular focus on plasma hemolytic indices. The outcome of this investigation is expected to provide insight into the potential antagonistic or synergistic interactions between Cd and Fe in hematological toxicity and may contribute to the development of protective strategies against Cd-induced anemia.
MATERIALS AND METHODS
Materials
Animals
A total of forty (40) adult male albino rats (Wistar strain), weighing between 160–180 g, were obtained from the Department of Animal Science, Obafemi Awolowo University, Ile-Ife, Osun State, Nigeria. The animals were acclimatized for one week prior to the commencement of the experiment.
Chemicals and Reagents
All chemicals and reagents used in the study were of analytical grade and procured from standard commercial sources.
Methods
Animal Care and Treatment
The animals were housed in well-ventilated wooden cages under standard laboratory conditions (12-hour light/dark cycle, temperature 25 ± 2 °C, relative humidity 50–60%). They had free access to standard rat chow and clean water ad libitum. All experimental procedures were carried out in strict accordance with the National Institutes of Health (NIH) guidelines for the care and use of laboratory animals (NIH Publication No. 85-93, revised 1985). Ethical approval was obtained from the Faculty of Life Sciences Research Ethics Committee (FLSREC), University of Benin (Approval No.: FLSREC-2023-007).
Preparation of Tainted Drinking Water
Cd and Fe were dissolved in distilled water to prepare tainted drinking water solutions based on the concentrations used in a previous study by Ogunbiyi et al. (2019).
Preparation and Treatment of Catfish (Clarias gariepinus)
Catfish used as the protein component in the feed was treated as outlined by Ogunbiyi and Obi (2022). Feed formulations containing Cd, Fe, or a combination of both were prepared in accordance with the protocol by Asagba and Obi (2005).
Experimental Design
The rats were randomly assigned into four (4) groups (n = 5 per group) and exposed to the treatments for two phases (via tainted drinking water in the first phase and tainted feed in the second phase) over a period of four weeks:
Experimental Group Treatment
A (Control) Rats exposed to Cd- and Fe-free drinking water/feed
B (Cd only) Rats exposed to Cd-tainted water/feed (0.229 mg/L)
C (Fe only) Rats exposed to Fe-tainted water/feed (1.900 mg/L)
D (Cd + Fe) Rats exposed to combined Cd and Fe-tainted water/feed
Sample Collection
At the end of the four-week exposure period, blood samples were collected from each rat via retro-orbital puncture under mild anesthesia into EDTA-coated tubes for hematological analysis.
Sample Preparation
Plasma Samples
Collected blood samples were centrifuged at 3,000 rpm for 15 minutes. The plasma supernatant was carefully aspirated and stored at –20 °C until further analysis for hemolytic indices.
Assessment of Hematological Indices
Haematological evaluations were performed using standard protocols. Red blood cells (RBC) and white blood cells (WBC) were counted using an improved Neubauer counting chamber (Doyle, 2006). The packed cell volume (PCV) was determined using the microhematocrit method, where centrifuged blood in capillary tubes was separated into red cells, buffy coat, and plasma layers.
Hemoglobin concentration (Hb) was measured using the cyanohaemoglobin method. This involves lysing erythrocytes to release hemoglobin, which is then converted to a stable cyanide derivative and quantified photometrically.
Differential leukocyte counts, including neutrophils, lymphocytes, monocytes, eosinophils, and basophils, were determined microscopically using standard smear staining and enumeration methods. Other red cell indices such as mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) were calculated using values derived from RBC count, Hb concentration, and PCV. An automated hematology analyzer was used for confirmatory measurements of complete blood counts and indices (Doyle, 2006).
Data Analysis
All data were expressed as mean ± standard error of the mean (SEM). Statistical comparisons among treatment groups were performed using one-way analysis of variance (ANOVA), followed by Tukey’s multiple comparison test to assess intergroup significance using GraphPad Prism version 6.0. Differences were considered statistically significant at p ≤ 0.05.
RESULTS
Haematological Parameters of Rats Exposed to Cadmium and Iron via Water and Feed
The haematological parameters of rats exposed to cadmium (Cd) and iron (Fe), either individually or in combination through tainted water and feed, are presented in Figures 1 to 10.
No significant differences (p > 0.05) were observed in packed cell volume (PCV), neutrophils (NEUT), lymphocytes (LYMPH), monocytes (MONO), eosinophils (EOS), and basophils (BASO) among treated groups compared to the control group.
However, red blood cell (RBC) count was significantly increased (p ≤ 0.05) in rats exposed to both Cd and Fe via water, compared to those exposed to Fe only. Conversely, white blood cell (WBC) count significantly decreased (p ≤ 0.05) in rats exposed to Fe only, compared to those exposed to Cd only or the combined Cd+Fe treatment (Figure 1).
Thrombocyte count was significantly reduced (p ≤ 0.05) in rats exposed to both Cd and Fe compared to those exposed to Cd alone via water. In rats exposed via feed, RBC and thrombocyte counts were significantly decreased (p ≤ 0.05) in the Fe-only group compared to controls (Figure 2).
Additionally, WBC counts were significantly lower (p ≤ 0.05) in all treated groups (Cd, Fe, and Cd+Fe) compared to the control when exposure occurred through feed. Among these, WBC levels in the combined treatment group were significantly lower than in the Fe-only group (Figure 2).
No significant alterations (p > 0.05) were observed in PCV, NEUT, LYMPH, MONO, EOS, and BASO among any of the groups exposed to Cd, Fe, or both via water or feed (Figures 3–6).
Haemoglobin concentration remained unchanged in rats exposed via water (Figure 7). However, haemoglobin levels were significantly reduced (p ≤ 0.05) in rats exposed to Cd alone compared to those exposed to both Cd and Fe via feed (Figure 8).
Mean corpuscular haemoglobin concentration (MCHC) was significantly decreased (p ≤ 0.05) in rats exposed to Cd only via water compared to the control group. Similarly, mean corpuscular volume (MCV) significantly decreased (p ≤ 0.05) in rats exposed to both Cd and Fe compared to Fe-only exposure via water (Figure 9). Conversely, MCV levels significantly increased (p ≤ 0.05) in rats exposed to Fe alone or Cd+Fe via feed.
Furthermore, mean corpuscular haemoglobin (MCH) was significantly elevated (p ≤ 0.05) in rats exposed to both metals via feed compared to rats exposed to Fe only (Figure 10).
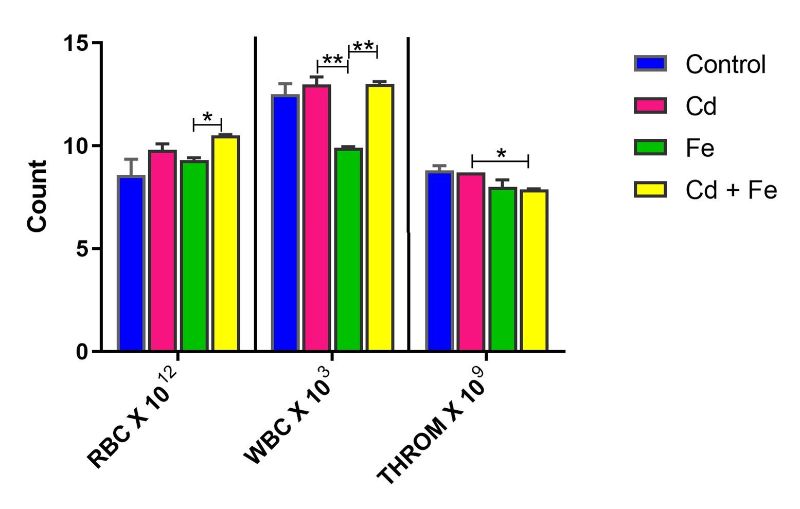
Figure 1. Red Blood Cell, White Blood Cell and Thrombocyte of rats exposed via water
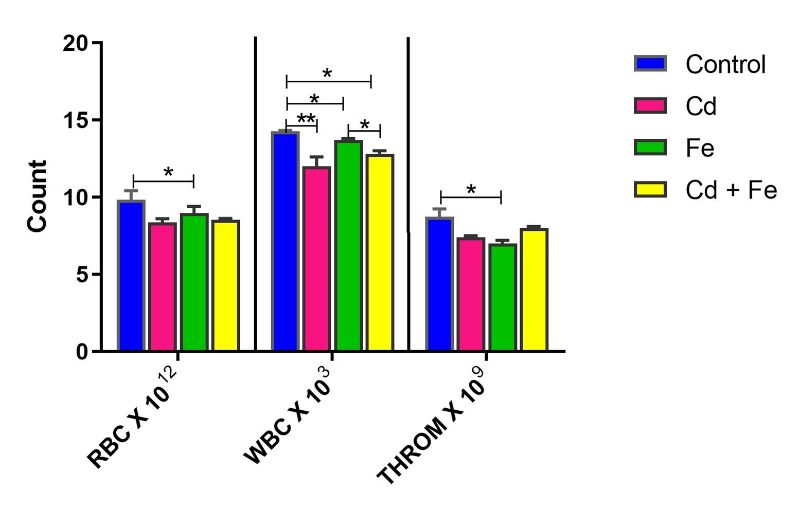
Figure 2. Red Blood Cell, White Blood Cell and Thrombocyte Counts of rats exposed via feed
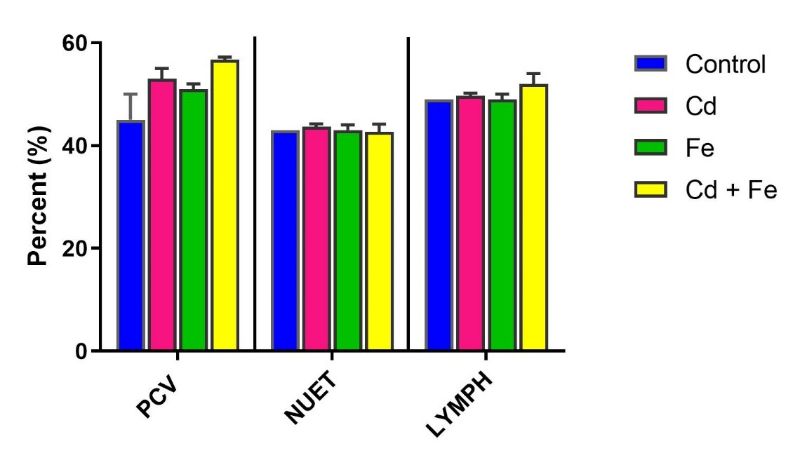
Figure 3. Pack Cell Volume, Neutrophils and Lymphocytes of rats exposed via water
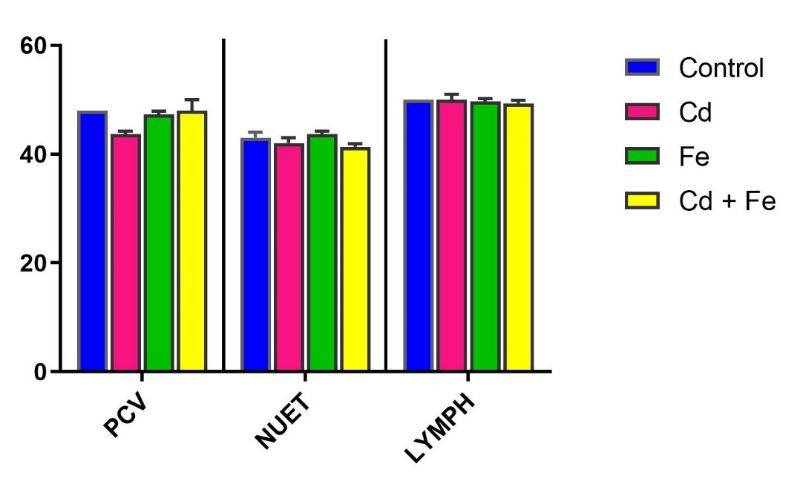
Figure 4. Pack Cell Volume, Neutrophils and Lymphocytes of rats exposed via feed
Figure 5. Levels of Monocytes, Eosinophils and Basophils of rats exposed via water
Figure 6. Levels of Monocytes, Eosinophils and Basophils of rats exposed via feed
Figure 7. Haemoglobin levels of rats exposed via water
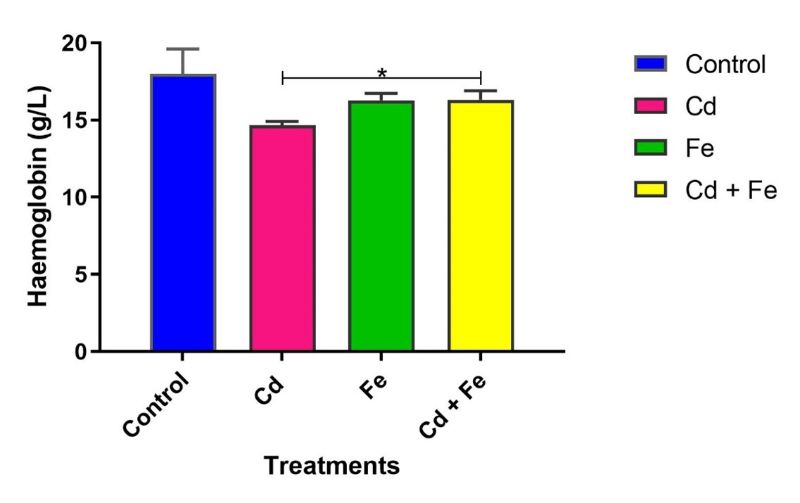
Figure 8. Haemoglobin levels of rats exposed via feed
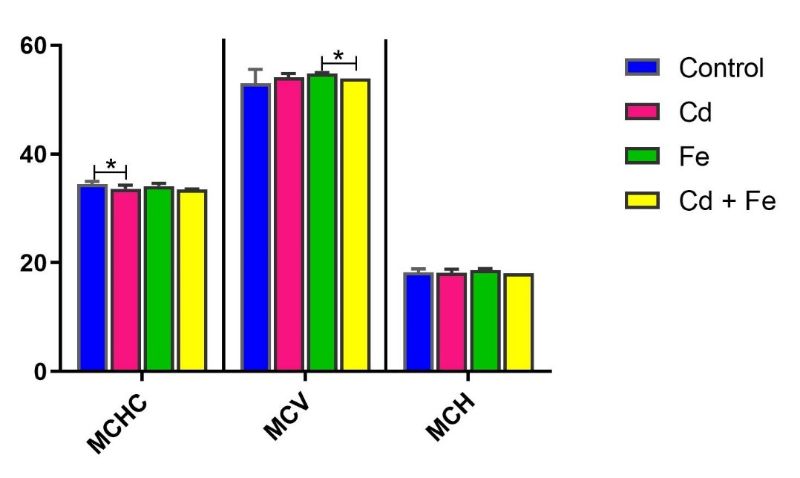
Figure 9. Mean Corpuscular Haemoglobin Concentration (g/dL), Mean Corpuscular Volume (fL) and Mean Corpuscular Haemoglobin (pg/cell) in rats exposed via water
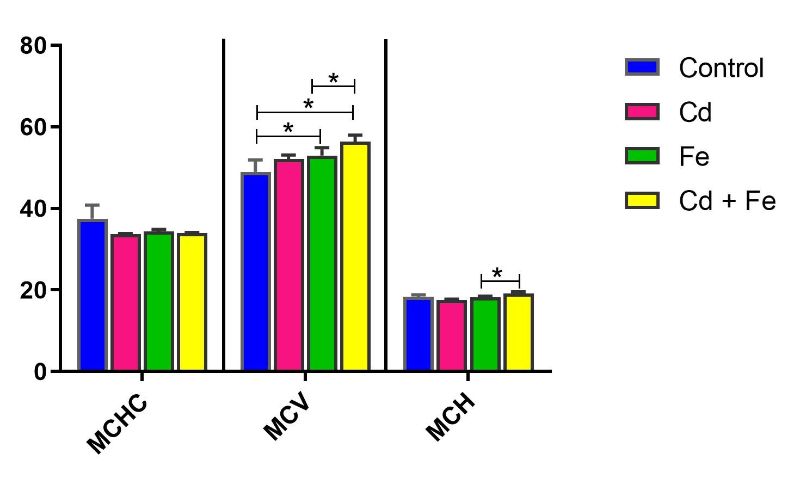
Figure 10. Mean Corpuscular Haemoglobin Concentration (g/dL), Mean Corpuscular Volume (fL) and Mean Corpuscular Haemoglobin (pg/cell) in rats exposed via feed
Note: *Values carrying asterisks are significantly different (p≤0.05) from one another, while those without asterisks do not differ significantly from one another.
DISCUSSION
Cadmium (Cd) and iron (Fe) are environmental metals known to induce a variety of biochemical physiological, and haematological dysfunctions in humans and animals (Santos et al., 2004; Husain, 2015; Yang et al., 2019; Njoku et al., 2025). This study evaluated the haemolytic effects of Cd and Fe, administered either singly or in combination via tainted drinking water and feed, on adult male rats.
The haematological results revealed that RBC, WBC, and thrombocyte counts were significantly decreased (p ≤ 0.05) in rats exposed to Fe only via feed, compared to control. This aligns with earlier reports indicating that excess Fe acts as a pro-oxidant, generating free radicals that may damage blood cells and impair haematopoiesis (Ho-Wang & Wenxia, 2023). The reduced WBC count also supports the notion that iron overload may suppress immune function, increasing susceptibility to infection (Shah et al., 2021).
Similarly, a significant decrease in WBC count was observed in rats exposed to Cd alone via feeding, corroborating findings by Mirkov et al. (2021), who reported that Cd exposure alters cellular immune responses. This immune suppression can compromise host defense mechanisms. Also, these findings are consistent with previous reports indicating that Cd exposure induces haematological abnormalities. For instance, Husain (2015) reported that exposure to Cd in growing rats led to structural anomalies in both erythrocytes and leukocytes, resulting in microcytic hypochromic anaemia, erythrocytopenia, and leukocytopenia. This supports the observed reduction in red and white blood cell counts in the present study and highlights the haematotoxic potential of cadmium, particularly its role in disrupting erythropoiesis and immune cell integrity.
Notably, rats exposed to Cd only via tainted water exhibited a significant decrease in MCHC, as well as decrease in haemoglobin level when exposed via tainted feed suggesting hypochromic anaemia and impaired haemoglobin synthesis. This is consistent with prior studies linking Cd exposure to reductions in haematocrit, RBC count, and haemoglobin levels in both aquatic organisms and rodents (Mekkawy et al., 2011; Nikolić et al., 2015; Sugiharto et al., 2020; Lee et al., 2022).
Conversely, an increase in MCV was observed in rats exposed to Fe and Cd + Fe via feed, possibly indicating macrocytic changes due to altered erythropoiesis. Increased iron availability might stimulate immature erythroid cell proliferation and haemoglobin production, as previously described by Barton et al. (2000).
Interestingly, exposure to both Cd and Fe appeared to mitigate some individual toxic effects, particularly the Cd-induced decrease in MCHC and Fe-induced suppression of WBC count. This suggests a potential interaction between the two metals that may modulate their respective toxicokinetic profiles and their haematological parameters. Exposure to Cd and Fe contaminated catfish has been recently reported to adversely affect some haematological parameters in rats (Njoku et al., 2025).
In general, this study demonstrates that exposure to Fe and Cd, especially via feed, such as consumption of catfish contaminated with these metals, can result in haematological alterations indicative of haemotoxicity. Fe alone significantly reduced RBC, WBC, MCV, and thrombocyte counts, while Cd alone affected WBC and haemoglobin level. However, combined exposure altered the expected toxicity patterns, suggesting possible antagonistic or adaptive responses in the presence of both metals.
CONCLUSION
This study demonstrated that both Cd and Fe, when administered via tainted water and feed, can alter haematological parameters in adult male rats. Iron exposure alone, particularly via feed, induced significant decreases in RBCs, WBCs, MCV, and thrombocyte counts, indicating potential haemotoxicity. Cadmium exposure also led to reductions in WBC and haemoglobin level, consistent with immune suppression and impaired erythropoiesis. However, concomitant exposure to both metals modulated some of these effects, with evidence suggesting that iron may mitigate certain toxic responses induced by cadmium, particularly in haematological indices. These findings suggest a complex interaction between Cd and Fe, where Fe may exert both toxic and protective roles depending on the exposure route and context.
RECOMMENDATION
Further toxicological studies are recommended to elucidate the dose-dependent effects and underlying mechanisms of cadmium and iron interactions on haematological parameters, with a focus on their potential synergistic or antagonistic toxicity when co-administered.