Aditum Journal of Clinical and Biomedical Research
OPEN ACCESS | Volume 8 - Issue 1 - 2026
ISSN No: 2993-9968 | Journal DOI: 10.61148/2993-9968/AJCBR
Mbarka Belboukhari, Fatiha Othmane, Nasser Belboukhari * , Khaled Sekkoum
Bioactive Molecules and Chiral Separation Laboratory, Faculty of exact sciences, Tahri Mohammed University, Istiklal street PO 417 Bechar, 08000, Algeria.
*Corresponding Author: Nasser Belboukhari, Bioactive Molecules and Chiral Separation Laboratory, Faculty of exact sciences, Tahri Mohammed University, Istiklal street PO 417 Bechar, 08000, Algeria.
Received Date: February 14, 2022
Accepted Date: March 08, 2022
Published Date: April 08, 2022
Citation: Mbarka Belboukhari, Fatiha Othmane, Nasser Belboukhari, Khaled Sekkoum. (2022) “ Chiral Analysis of Amlodipine by Hplc Methods.”, Aditum Journal of Clinical and Biomedical Research. 4(2) ; DOI: http;//doi.org/03.2022/1.1073.
Copyright: © 2022. Nasser Belboukhari. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly Cited.
Amlodipine is a cardiovascular drug commonly used as a sole treatment for high blood pressure or it can be combined with other antihypertesive agents. In this work carried out at the level of bioactive molecules and chiral separation laboratory of matter sciences at university tahri Mohammed Béchar .we stud we studied the chiral separation by CLHP of (amlodipine 5mg) in the normal-phase mode and organic polar mode using six polysaccharide-derived chiral stationary phases (CHIRALCEL OD-RH, CHIRALCEL OD-3R, CHIRALPAK IA) and C18. Or at the same time we understand all the principles of the modules constituting a CLHP Schimadzu LC-2030 system and the characteristics of CLHP.
1. Introduction:
Amlodipine is a chemical substance with very specific physico-chemical and pharmacological properties. It remains a model molecule in biomedical and pharmacological research. There are about 80 salts of amlodipine, the best known of which are: maleate, tozelat, besylate, etc.[1]
In the medical field, the selection of a salt is a long and difficult operation. In this sense, the pharmaceutical company Pfizer had used amlodipine maleate in the manufacture of pharmaceutical specialties, but later discovered that it did not lend itself to formulation in an adequate dosage form, due to a problem significant stability, which to solve it, it was necessary to replace it with a new salt of amlodipine. Thus, it was in 1987 that Pfizer discovered amlodipine besylate, as a new bioactive molecule, which has very important pharmaceutical properties compared to the maleate form.[2]
Amlodipine besilate is a calcium antagonist. It is indicated in the treatment of certain cardiovascular disorders, in particular arterial hypertension and angina pectoris (or angina). Marketed by several laboratories (pfizer, Aventis, Txeva...), it comes in the form of a tablet and is administered orally. It is sometimes combined with other active ingredients (such as: valsartan, olmesartan, telmisartan, atorvastatin). The most common side effects associated with amlodipine are headache [3].
Amlodipine besylate is a cardiovascular drug commonly used as a single treatment for high blood pressure or it can be combined with other antihypertensive agents [4].
The recommended doses for a patient using this medication may be 5mg or 10mg, once a day.
But in the case of an elderly patient or a patient who has liver problems, the dose should be reduced to 2.5mg, because this patient may be easily exposed to the risk of accidents due to another combination therapy, which may cause
side effects [5].
With the chemical formula (C26H31ClN2O8S), and also named: Benzene sulfonate of (4RS)-2-[(2aminoethoxy)-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate of 3 - ethyl
and 5-methyl [6], is an inhibitor of the entry of calcium ions into the cell (calcium antagonist or calcium channel blocker), from the class of 1, 4 dihydropyridines [7].
It comes in the form of a white powder, it is sparingly soluble in water, easily soluble in methanol and quite soluble in ethanol, its molar mass is 567.1 g/mole-1 [8], and its chemical formula is shown in figure 1.
Figure 1: Chemical structure of amlodipine
Amlodipine besylate, like all members of the 1,4-dihydropyridine calcium channel blockers, is photosensitive and susceptible to degradation both in solution and in the solid state.
Light catalyzes its oxidation to pyridine derivatives, such as amlox (2-a(2-aminoethoxy) Hyl-4-(2-chlorophenyl)-3-ethoxycarbonyl-5methoxycarbonyl-6 methylpyridine) which lacks effects. therapeutic Forced degradation studies show that amlodipine degrades slowly under thermal effect (more in solution than in solid state), degrading faster under photo-stress and even more so under acid, alkaline and oxidative stress [9].
The aim of our experimental work is to study the separation of enantiomers and diastereoisomers of amlodipine by HPLC methods in two chromatographic modes: in normal phase and in polar organic phase [10-15]. We will thus present in this work the results of chiral separation of amlodipine carried out in our laboratory. After achieving complete chiral resolution, the calculation of chromatographic factors is very essential.[16]
The most important factors to calculate are the retention factor (k'), the separation factor (α), and the resolution factor (Rs) and the peak areas for the resolved enantiomers [17,18]. The values of these parameters can be calculated by operating software (Shimadzu® LC solution).[19]
2. Materiels And Methods:
2.1. Reagents:
Amlodipine pure drug has been purchased from USP, Twinbrook, Pkway, Switzerland. The Acetonitril (CAN), Isopropanol and n-hexane HPLC grade were supplied from Sigma -Aldrich and Riedel-de Haën (Sleeze, Germany).
2.2. Apparatus:
UV-VIS : UV-VIS spectra of Amlodipine are recorded on SPECORD 200 PLUS-223E1121 at the range of 190–800 nm, with scanning speed 10 nm/s, using methanol as blank solvent, also as solvent for all compounds, the measurements were performed at room temperature.
2.3. HPLC instrumentation:
All HPLC experiments were performed witha SHIMADZU LC 20-A instrument equipped with a vacuum degasser, PerkinElmer (Norwalk, CT,USA), Shimadzu®LC 20 AD (Kyoto, Japan) 200 LC pump, injectorwith 20 µL Rheodyne 1907 sample loop equipped with a UV detector Shimadzu SPD-20 A (Kyoto, Japan).
3. Results and Discussions:
3.1. Analysis of Amlodipine by UV-Vis Spectroscopy:
The UV-Visible absorption spectrum of amlodipine (Figure 2). recorded in water shows three characteristic absorption bands.
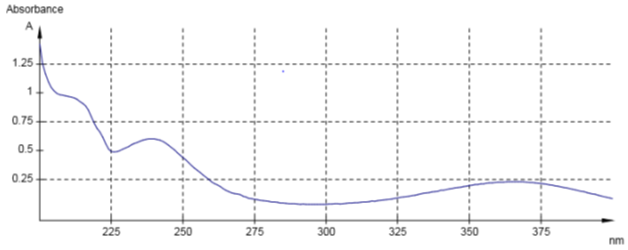
Figure 2: UV-Visible absorption spectrum of amlodipine recorded in distilled water.
According to the Beer-Lamber absorption law, the molecular extinction coefficients can be determined, which gives information on the intensities of the absorption bands. The first absorption band is located at max=211nm with a very strong intensity around 0.6640 corresponds to electronic transitions of the π-π* type with energy of 135.62 kcal/mol. Corresponding to the double bonds (C=C) of the two aromatic rings.
The second band is located at lmax=238nm with an intensi0ty of 0.4121 attributed to electronic transitions of the π-π* type with energy of 120.24 kcal/mol. corresponding to double lessons (C=O). The third absorption band is at lmax=364nm with an intensity of 0.1565 corresponds to n-π* type electronic transitions with energy of 78.62 kcal/mol corresponding to free doubles (CO), (CN), (C=O).
3.2. HPLC-UV analysis of amlodipine on C18 column :
The analysis of amlodipine on the C-18 column was carried out under the following conditions: Mobile phase (Isopropanol 50٪, Hexane50٪), flow rate (0.5ml/min-1), injection volume (10μl) and detection (238 nm).
We note from the results of analysis by HPLC on the C-18 column shows that this product appears at 4.409 min in 89.5% and peak 2 (Figure 2) at 5.787 min attributed to the excipient and since this analysis was carried out with detection at a wavelength of 238 nm where the amlodipine peak has a weak absorption which escapes the exploration of the other transparent excipient peaks in this detection position.
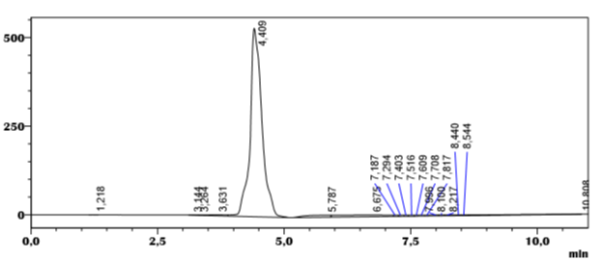
Figure 3: Chromatogram of the separation of amlodipine by HPLC on the C18 column (Isopropanol 100%).
3.3.Chiral analysis of amlodipine:
For the CHIRACEL OD-RH column, the chiral separation of amlodipine shows the presence of four constituents eluted respectively at 4.119 min, 5.074 min, 5.929 min, 9.469 min with strong resolutions around: 1.895, 2.955 (Table 1) at gradient mode of isopropanol and hexane (40/60), with a flow rate of .05ml/min, the chromatogram shows the presence of the first two peaks located respectively at TR = 4.119 min and 5.074 min with average resolution (Rs = 0.158) (Table 1).
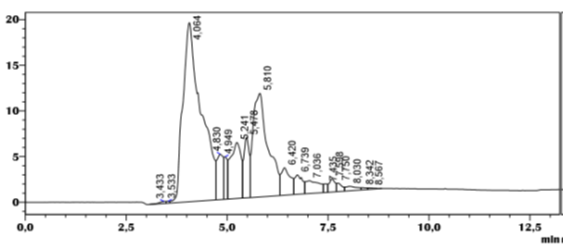
Figure 4: chromatogram of the separation of amlodipine by HPLC on the CHIRACEL OD-RH column, Eluent (Isopropanol/Hex 50/50).
Analysis of amlodipine on the Chiralpak IA column with hexane and isopropanol (50/50) as the mobile phase shows the existence of six constituents, with a retention time around 3.90 min, 4.05 min, 4.62 min,4.96 min,5.30 min, 5.69 min (Table 1) with a low resolution around Rs = 0.88, and a good selectivity value varies between (1.27 to 4.61) (Table 1), (Figure 4).
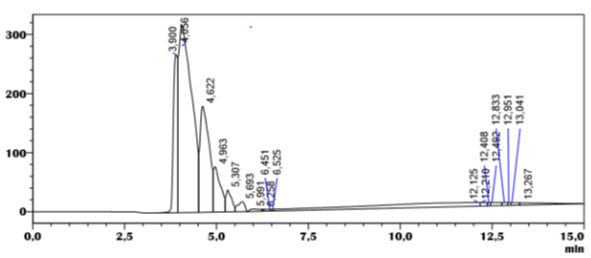
Figure 5: chromatogram of the separation of amlodipine by HPLC on the column, Chiralpak IA, Eluent (Isopropanol/Hex 50/50).
For the CHIRACEL OD-3R column, the chiral separation of amlodipine shows the presence of four constituents eluted respectively at 1.33 min, 2.40 min, 2.73 min, 4.78 min and it is observed that these peaks have acceptable selectivity factors varying between 1.69 and 1.07, on the other hand, these peaks have low resolutions between 0.12 and 0.01 (Table 1) in the gradient mode of isopropanol and hexane (40/60), with a flow rate of .05ml/min (Table 1), (Figure 6).
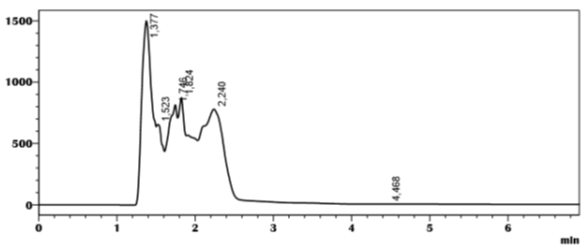
Figure 6: chromatogram of the separation of amlodipine by HPLC on the CHIRACEL OD-3R column, Eluent (Hexane/Ethanol (40/60).
|
PSC |
Eluent |
Pic |
tR |
k’ |
α |
Rs |
% |
|
CHIRACEL OD-RH |
IPrOH/Hexan (50 :50) |
1 |
4.06 |
0.11 |
- |
- |
81.05 |
|
2 |
4.83 |
0.13 |
1.18 |
0.842 |
10.82 |
||
|
3 |
4.94 |
0.21 |
1.6 |
0.274 |
3.12 |
||
|
4 |
5.24 |
0.24 |
1.14 |
0.100 |
3.56 |
||
|
5 |
5.47 |
0.27 |
1.12 |
0.050 |
1.45 |
||
|
IPrOH/Hexan (40 :60) |
1 |
4.11 |
0.19 |
- |
- |
67.05 |
|
|
2 |
5.07 |
0.23 |
1.21 |
0.518 |
24.75 |
||
|
3 |
5.92 |
0.43 |
1.89 |
0.296 |
6.86 |
||
|
4 |
9.46 |
1.29 |
2.95 |
1.416 |
1.34 |
||
|
CHIRAL PAK IA |
IPrOH/Hexan (50 :50) |
1 |
3.90 |
0.038 |
- |
- |
14.84 |
|
2 |
4.05 |
0.04 |
1.05 |
0.885 |
53.01 |
||
|
3 |
4.62 |
0.18 |
4.50 |
0.694 |
21.08 |
||
|
4 |
4.96 |
0.27 |
1.50 |
0.435 |
6.88 |
||
|
5 |
5.30 |
0.36 |
1.33 |
0.496 |
2.70 |
||
|
6 |
5.69 |
0.46 |
1.27 |
0.883 |
1.49 |
||
|
ACN 100% |
1 |
4.70 |
9.67 |
- |
- |
3.08 |
|
|
2 |
4.79 |
9.87 |
1.02 |
0.504 |
27.69 |
||
|
3 |
5.23 |
12.19 |
1.23 |
0.057 |
12.30 |
||
|
4 |
5.43 |
16.92 |
1.38 |
0.114 |
30.77 |
||
|
5 |
6.36 |
18.63 |
1.10 |
0.012 |
7.69 |
||
|
6 |
6.48 |
20.05 |
1.07 |
0.010 |
18.47 |
||
|
CHIRAVEL OD-3R |
Hexan/Ethanol (40 :60) |
1 |
1.37 |
0.099 |
- |
- |
26.65 |
|
2 |
1.52 |
0.11 |
1.11 |
0.193 |
7.15 |
||
|
3 |
1.74 |
0.26 |
2.36 |
0.265 |
13.09 |
||
|
4 |
1.82 |
0.32 |
1.21 |
0.080 |
19.02 |
||
|
5 |
2.24 |
0.62 |
1.93 |
0.378 |
33.97 |
||
|
6 |
4.46 |
2.24 |
3.58 |
1.792 |
0.09 |
||
|
IPrOH/Hexan (40 :60) |
1 |
1.33 |
1.33 |
- |
- |
43.75 |
|
|
2 |
2.40 |
1.61 |
1.21 |
0.117 |
12.5 |
||
|
3 |
2.73 |
1.93 |
1.19 |
0.019 |
12.5 |
||
|
4 |
4.78 |
2.06 |
1.07 |
0.001 |
6.25 |
||
|
5 |
4.56 |
2.24 |
1.08 |
0.001 |
18.75 |
||
|
6 |
5.11 |
3.81 |
1.69 |
0.125 |
6.25 |
Table 1: Results of chiral analysis of amlodipine by HPLC methods on three CSPs
(CHIRACEL OD-RH, CHIRAL PAK IA, CHIRACELOD-3R) , FR =0.5 ml/min
3.4. Comparison between the efficiency of the three columns in the chiral separation of amlodipine:
According to the results grouped in the tables, if we compare the efficiency of each column for amlodipine, we note that for the CHIRACEL OD-3R column, the amlodipine is too selective compared to the CHIRACEL IA and CHIRACEL OD-3 columns. RH with isopropanol and hexane (60/40) as a mobile phase, this method allows us to separate four isomers, a number greater than the number of isomers separated by the CHIRACEL OD-RH column, so this method allows us to to obtain separations with good selectivity factors and to have peaks with good resolution values (Table 1).
4. Conclusion :
Chiral analysis of amlodipine drug by HPLC methods shows the separation of 4 to 6 constituents where four out of the six constituents may be two enantiomers due to the presence of an asymmetric carbon in the structure and two atropoisomers due to blockade of the free rotation in the axis that binds the two aromatic rings.
5. Acknowledgment:
This work is supported by the General Direction of Scientific Research of Algeria (DGRSDT), and the authors are grateful to ministry of higher education and scientific research.