OPEN ACCESS | Volume - Issue -
ISSN No: | Journal DOI:
Niza Rean Simwanza
The Copperbelt University School Of Graduate Studies Micheal Chilufya Sata School Of Medicine.
*Corresponding author: Niza Rean Simwanza, The Copperbelt University School Of Graduate Studies Micheal Chilufya Sata School Of Medicine.
Received: March 09, 2025
Accepted: April 24, 2025
Published: May 04, 2025
Citation: Niza R Simwanza. (2025) “Knowledge, Attitude And Practice Regarding Pulmonary Tuberculosis As An Occupational Health Disease Among Miners At Neelkanth In Ndola, Zambia, Journal of International Research and Reviews. 1(1); DOI: 10.61148/JIRRS/003.
Copyright: © 2025 Niza Rean Simwanza. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Zambia is one of the many countries in sub-Saharan Africa that is burdened by tuberculosis (TB). Mine workers in Southern Africa including Zambia tend to have poor living and working conditions thereby having increased risk of TB and in addition working in the mines increases exposure to silica dust leading them to developing silicosis which increases their risk of developing PTB. Furthermore, ex-miners also tend to have an increased risk of TB due to previous exposure to silica dust.
The general objective of this study was to assess the knowledge, attitude and practice regarding TB as an occupational health disease among miners at Neelkanth mine in Ndola.
Methodology
This was a cross sectional study that assessed the Knowledge, Attitude and Practices of miners regarding TB as an occupational health disease. The study was conducted at Neelkanth mine in Bwana M’kubwa area, Ndola rural. Study participants were miners. The calculated sample size for the study was 384. A questionnaire was used to collect data from study participants. Data was entered and analysed using Spss version 16.0, Pearson chi squared test was performed and the output was then analysed further using multivariate logistic regression at 95% confidence interval.
Results
This study resulted in a total of 357 study participants instead of the calculated 384 that wereenrolled into the study, due to the fact that 27 questionnaires were incomplete and therefore eliminated from the study. The mean age was calculated to be 32.9 (standard deviation [SD]:7.4) years, with the majority of participants aged between 18-40years. Comparing the participants that could define TB to those that could not, those that could define were 1.84 times more likely to have good knowledge levels (CI95: 1.17, 2.91). Likewise, participants were 1.66 times more likely to know preventive measure of TB as an occupational health hazard compared to those who did not know (CI95: 1.18, 2.32). Participants who wear PPE were more than twice likely to protect themselves from TB compared to other study participants who did not (CI95: 1.25, 2.34). Participants who do not go for regular chest examination were more than twice likely to have TB compared to the study participants whodid not (CI95: 1.13, 2.11).
Conclusion
This study revealed through multivariate regression analysis of the results that there is a significant association between knowledge, attitude, practice and TB as an occupational health disease. These findings highlight the need for TB education amongst miners.
Introduction
Tuberculosis is an infectious disease caused by the bacteria Mycobacterium tuberculosis. The bacteria spreads through the air from person to person and mainly attacks the lungs, but it can affect other areas of the body [1]. The majority of people exposed to the bacteria do not experience tuberculosis symptoms right away [1]. Instead, the infection may go through three stages:
Primary TB Infection; this is when the bacteria first enter your body. In many people this causes no symptoms, but others may experience fever or pulmonary symptoms [2]. Most people with a healthy immune system will not develop any symptoms of infection, but in some people the bacteria may grow and develop into an active disease. Most primary TB infections are asymptomatic and followed by a latent TB infection [2].
Latent TB Infection; The bacteria are in your body and can be found through tests, but is not active. During this stage you do not experience symptoms and cannot spread the disease to others [3].
Active Disease; the TB bacteria are active and multiplying. You will feel sick and will be contagious. It is important to seek immediate treatment to avoid complications and infecting others [3].
 Figure 1: shows Mycobacterium tuberculosis. Source; www.textbookofbacteriology.net
Figure 1: shows Mycobacterium tuberculosis. Source; www.textbookofbacteriology.net
A third of the world's population is infected with Mycobacterium tuberculosis (MTB), and over 9 million new cases of tuberculosis (TB) are reported annually [4]. Treatment of drug- susceptible pulmonary TB is highly effective, with 85% (66 million cases) of reported cases estimated to have been successfully treated between 1995 and 2015 [4]. However, up to half of TB survivors have some form of persistent pulmonary dysfunction despite microbiologic cure [5-8]. Pulmonary dysfunction, ranging from minor abnormalities to severe breathlessness, can increase the risk of death from respiratory causes [9, 10].
Furthermore, treated TB patients appear to contribute substantially to the growing worldwide burden of chronic obstructive pulmonary disease (COPD) [9–10].
The symptoms of TB include a low-grade fever, night sweats, fatigue, unexplained weight loss of ten pounds or greater and a persistent cough [10]. Some people may not have obvious symptoms. Evidence of infection (a positive skin test) may occur from two to 10 weeks after exposure. The most hazardous period for developing TB disease is usually within six to 12 months after infection, but can be longer, occurring much later in life. [10].
The term ‘pneumoconiosis’ is used to describe a set of lung diseases caused by the repeated inhalation of small particles in which long-term retention in the lung is a key causative factor. [11]The site of damage within the lung is a function of both the size and the toxicity of the inhaled dust, fume or fibre. The ability of different types of particles to cause fibrosis varies widely: crystalline silica is highly fibro genic, whereas iron oxide is not [11]. In susceptible individuals, pneumoconiosis usually develops after many years of exposure, sometimes presenting after retirement. [12] Where cases are detected during employment (e.g. during health surveillance), reduction or cessation of further exposures should be the goal. Prevention is of prime importance as the lung damage caused by pneumoconiosis is irreversible, and the retained substance may continue to cause harm many years after exposure has ceased [13].
According to the Occupational Health and Safety Institute, the regulatory body established under the Occupational Health and Safety Act, 2010, of Zambia [14], the weighted average of the incidence rate of PTB among current miners 1994–2014 was 658 per 100 000 populations. Figure 2 shows descriptive data of a sample of the incidence rate of PTB among current copper miners. The incidence rate increased from 1994 to 2005 but since 2005, it has steadily decreased. In 2014, the incidence rate of PTB among miners in Zambia was 288 per 100 000 populations.
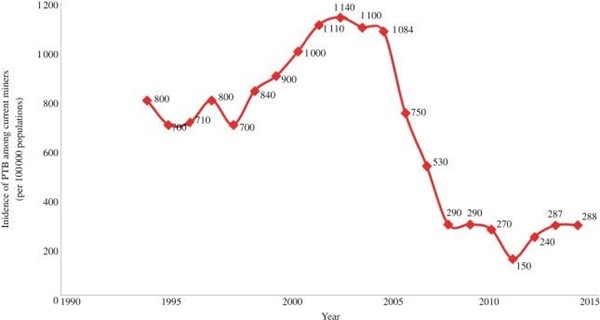
Figure 2: Trends of PTB incidence among current copper miners based on Occupational Health and Safety Institute (Kitwe, Zambia) records review. Source; Tuberculosis in the mines of Zambia: A case for intervention by Pascalina Chanda-Kapata and colleagues.
The TB notification rate is the number of TB cases (new and relapse) notified to the national health authorities during a specified period of time per 100,000 populations [97]. The TB notification rate as reported by the Zambian Ministry of Health through the National TB Control Program may be used as an indicator to determine the magnitude of the burden of TB in a specific location [15]. The two main provinces where the mines are concentrated had the highest TB notification rates. The Copper Belt Province which holds the most copper mines in the region had a notification rate of 415 per 100 000 people in 2013 which was more than 10- fold the national TB notification rate [15].
Statement Of The Problem
The national prevalence survey showed the Copper Belt Province to have the highest prevalence of bacteriologically confirmed TB of 1211 per 100 000 people in the general population. Therefore, it was the aim of this research to explore the factors leading to this confirmed prevalence rate, in Ndola rural, Bwana Mkubwa area, the site of Neelkanth mine. This site became an area of interest to be considered for studies regarding TB, so as to determine the knowledge, attitude and practice about TB as an occupational health hazard among the said miners. The miners at this mine is that they are working without proper safety boots, work suite or aspirators during their long shifts and this prompted this study.
Justification
The key to controlling TB in the mining sector is primary prevention based on comprehensive population-wide programmes that teach people on the importance of proper protective equipment while they work as well as proper adherence to treatment if found with TB, this is very important for every miner to know.
It was observed when a visit to Neelkanth a mine that is mining lime and electric cables in Bwana Mkubwa area, revealed that the miners there work without the full proper protective gear that is needed in such an environment. This study is important because its findings will reveal how knowledgeable the miners are about TB, the attitude and practices that they do in order to avoid putting themselves at the risk of contracting TB. It will also provide information to relevant authorities like Ministry of Health and other authorities that are concerned about TB in the mining sector with the gaps that need attention both on the mine management side and miner’s side.
Literature Review Global Review
Global TB targets for the period 2016–2035 have been set as part of the United Nations (UN) Sustainable Development Goals (SDGs), the World Health Organization (WHO) End TB Strategy and the political declaration of the UN high-level meeting on TB held in 2018[17]. The End TB Strategy was adopted by all WHO Member States at the World Health Assembly in 2014 [18]. The political declaration of the UN high-level meeting on TB held in September 2018 [19] reaffirmed the SDG and End TB Strategy targets. It also established new targets for the numbers of people to be provided with TB treatment and TB preventive treatment during the period 2018–2022, which were derived from and consistent with End TB Strategy milestones; and new targets for the funding to be mobilized between 2018 and 2022, based on the Stop TB Partnership’s Global Plan to End TB [20]. Globally, the TB incidence rate is falling, but not fast enough to reach the first milestone of the End TB Strategy; that is, a 20% reduction between 2015 and 2020. Worldwide, the cumulative reduction from 2015 to 2019 was 9% (from 142 to 130 new cases per 100000 population), including a reduction of 2.3% between 2018 and 2019 [21].
A total of 78 countries are on track to reach the 2020 milestone. This includes seven high TB burden countries1that have already reached it (Cambodia, Ethiopia, Kenya, Namibia, the Russian Federation, South Africa and the United Republic of Tanzania) and three others that are on track (Lesotho, Myanmar and Zimbabwe) [22]. Worldwide, TB is the leading infectious disease killer and one of the top10 causes of death overall [23].
Globally, some of the main key risk factors of TB include; Bacillary Load-Epidemiological studies conducted during mid- 20th century have shown that smear positive cases are more infectious than the others [24-25]. An untreated sputum positive patient can infect approximately 10 individuals per year, and each smear positive case can lead to two new cases of TB, at least one of which will be infectious [26-27]. Proximity to an Infectious Case. Close contacts of infectious TB cases including household contacts and care givers/health care workers [28] area at higher risk of becoming infected with Mycobacterium tuberculosis and development of primary active tuberculosis. Diabetes; Diabetes has been shown to increase the risk of active TB disease [29, 30]. It is estimated that currently 70% of people with diabetes live in low- and middle-income countries [31], and the rates are steadily increasing in areas where TB is endemic, including India and sub- Saharan Africa [32].
Alcohol; Alcohol has been recognized as a strong risk factor for TB disease [33]. The harmful use of alcohol ranks among the top five risk factors for disease, disability, and death, as well as being a causal factor in more than 200 disease and injury conditions, including tuberculosis, worldwide [34]. It has been estimated that approximately 10% of all tuberculosis cases are attributable to alcohol use [35]. Malnutrition; Studies have shown that malnutrition (both micro- and macro-deficiency) increases the risk of TB because of an impaired immune response [36–38]. TB disease can itself lead to malnourishment because of decreasing appetite and changes in metabolic processes [39]. The association between malnutrition and TB has been shown with BCG vaccine trials performed in USA during the late 1960s estimating that malnourished children are twice as likely to contract TB disease as their appropriately nourished peers [40]. Indoor Air Pollution; in developing countries, the percentage usage of solid fuels for cooking is more than 80% [41]. Firewood or biomass smoke has been previously recognized as an independent risk factor for TB disease in case control studies conducted in India and Brazil [42– 44]. Illicit drug use; Epidemiological data suggest that the relationship between tuberculosis and illicit drug use is increasing, leading to a public health problem because it involves political, human, social, and economic aspects [45-46]. Smoking; it is estimated that, worldwide, 1.3 billion people consume tobacco and that most of them live in underdeveloped or developing countries, where the tuberculosis rates are also higher [47]. Therefore, the greatest impact of smoking in terms of public health issues related to infection is probably the increase in the risk of tuberculosis. Some systematic reviews and meta-analyses of observational studies have shown an unfavourable association between the global epidemics of tuberculosis and smoking, exposure to tobacco smoke having been associated with tuberculosis infection, active tuberculosis, and tuberculosis- related mortality [48-49].
Mineral mining is one of the world’s most hazardous occupations, not only because of the safety issues involved, but also because of the clear link between mining, lung disease and TB [84–86]. Mineral mining exposes workers to high levels of silica dust [87], which carries with it an increased risk of lung disease [88-89], such as silicosis. Like HIV, silicosis has been demonstrated to greatly increase the risk of TB, including active TB [84]. The impact of silicosis on health has been known since the late 19th century. Even without the presence of silicosis, silica exposure alone is associated with an increased lifelong risk of TB disease [84- 87]. Data from India [86], China [88, 90] and Japan [91] have indicated that coal mining and residing in communities near coal mines might carry an increased risk of TB. Rural mining communities and miners’ families are susceptible to TB because of the circular migration patterns of miners as they travel between mines and their home [85, 92].
The KAP study done in Southern Africa revealed that; Knowledge on TB was universal across the ten SADC countries as 100% of the participants in the study, regardless of employment category, had received information on TB [98]. However, only 78% of the participants reported that they were aware of the main sources of TB information. This awareness ranged from 53% in Zimbabwe to 92% in Mozambique [98]. Compassion towards people with TB was high, with 78% of the respondents at regional level feeling that they would feel compassionate towards people with TB [98]. The data show very good health seeking behaviour with about 98% reporting that they would visit a health facility if they are found to have TB. There is minimal variability between countries and population types [98].
Africa Review
In Africa, tuberculosis is often the first manifestation of HIV infection, and it is the leading cause of death among HIV-infected patients [50–54]. In hospital-based series, 40–65% of HIV-infected African patients with respiratory disease had tuberculosis [24-26]. In primary health and chest clinic settings, tuberculosis was confirmed in 43–70% of adults with cough for 3 weeks or longer (chronic cough) in Zimbabwe, Kenya, and Malawi [30-31].
Figure 3: showing number of people provided with TB treatment. Source; global community report
Patients with tuberculosis now commonly present with atypical symptoms: MTB was isolated from 9% of adults with acute pneumonia in Kenya [32], 35% of people with cough for less than 3 weeks in Malawi [33] and 23% of febrile HIV-infected inpatients in Tanzania [34] and 13% of HIV-infected patients with chronic diarrhoea in Kenya [35].
In Africa, the following are some of the risk factors of TB in the mining sector. Poor living and working conditions further that exacerbate the risk for TB. A significant number of miners are migrants, traveling between their rural home areas and densely populated mining communities. More than 100,000 workers in the mining industry in South Africa come from Lesotho, Swaziland, Mozambique, or Botswana, and approximately 80 percent are concentrated in the gold mining industry (76). Deep-level gold mine shafts are often only two to three meters wide and can reach up to several kilometres underground (77). This makes crucial TB preventative strategies, such as air circulation and ventilation aimed at reducing silica dust, extremely difficult to implement, particularly in the cramped confines at the rock face [77]. Personal protection equipment, required to enter the mine shaft, is little used in practice because of poor enforcement and because workers often remove the equipment as a result of the increased difficulty breathing in a hot, sweaty environment (73). In part because of these conditions, it is estimated that 89 percent of miners are latently infected with TB (74). These individuals collectively represent a large population of individuals who develop active TB. Not only are rates of new TB infections high, but the rate of recurrent TB infection in the gold mining industry, whether or not complicated by silicosis, is more than twice the rate in the general population. Charalambous and colleagues showed that among 609 gold mine workers followed for a median of 1.02 years after a first cured episode of TB, 57 developed recurrent disease. Re-infection was demonstrated by genotyping in 69 percent of those with isolates recovered from both the first and subsequent TB episodes (80–82).
KAP studies done in Ethiopia revealed that; among the participants, 345 (83%) had heard information about TB [99] and One hundred seventy (41%) of participants responded the mode of transmission as through the air when a person with TB sneezes or cough, the majority (82%) said TB can be transmitted from person to person [99]. The majority (70%) of participants responded that its transmission is not preventable, while 92 (22.4%) replayed that covering the mouth and nose while coughing or sneezing is a possible method to prevent the transmissions [99]. The majority of participants (29%) considered TB as not very serious [99] and re than three-fourth (82%) of participants were have not received any health education about TB and 352 (85%) respondents did not take care during coughing or sneezing [99]. In Tanzania, study participant scoring at least 60% of the possible maximum scores was considered as having a good knowledge, positive attitude or good practices. And herein, a participant having positive TB attitude would mean they acknowledge TB exist, recognizes its impact on health and would seek or advise TB-infected individuals to seek the correct remedies [100].
National Review
Zambia is one of the many countries in sub-Saharan Africa that is burdened by tuberculosis (TB). The recent Zambia National TB Prevalence Survey 2013–2014 estimated the prevalence rate of all forms of bacteriologically confirmed pulmonary TB (PTB) among those aged 15 years and above to be at 638 per 100 000 populations which is higher than the prevalence rate in high TB burden countries such as Pakistan and Nigeria [37].
Approximately 70% of people with TB in Zambia are also co- infected with HIV [38]. According to the Zambia Demographic and Health Survey 2013–2014, the prevalence of HIV was estimated to be 13.5% in Zambia [39]. Before the HIV/AIDS epidemic in Zambia, the case notification rate of TB in Zambia in 1985 was 124 per 100 000 but the increasing HIV/AIDS epidemic in the period between 1990 and 2000 may have contributed to the increase of case notification rate to 512 per 100 000 as captured from routine facility based records countrywide [40]. The implementation of direct observed treatment short course and the TB/HIV collaborative control activities have contributed to a slight decline of TB [38].
However, the disease still remains a public health problem in Zambia [41-42]. TB being an airborne disease entails that enclosed areas such as mining sites with poor ventilation create favourable environments for TB transmission [43-44]. TB is one of the main health risks which have been found to be associated with mining [45].
Mine workers in Southern Africa including Zambia tend to have poor living and working conditions thereby having increased risk of TB and in addition working in the mines increases exposure to silica dust leading them to developing silicosis which increases their risk of developing PTB [93]. In addition, ex-miners also tend to have an increased risk of TB due to previous exposure to silica dust [47].
Underground mining produces dust, noise and in some mine sections heat and gases, such as sulphur dioxide. Workers also face risks of pneumoconiosis due to poor ventilation, and of rock falls causing injuries that may be fatal. These mines also raise the risk of contamination of water from the chemicals used in processing the minerals and acid mine drainage. These conditions were recorded in Nkana underground mine [93].
KAP studies on TB in the mines of Zambia proved to be scarce [17], however, other similar studies regarding TB in the mines were considered in this literature review as stated above
General Objective
To assess the knowledge, attitude and practice regarding TB as an occupational health disease among miners at Neelkanth mine in Ndola.
Specific Objectives
1. To determine the knowledge levels among Neelkanth miners regarding TB as an occupational health disease.
2. To determine the attitude among Neelkanth miners regarding TB as an occupational health disease.
3. To assess the practices among Neelkanth miners regarding TB as an occupational health disease.
Research Questions
1. What are the knowledge levels among the Neelkanth miners regarding TB as an occupational health disease?
2. What is the attitude among Neelkanth miners regarding TB as an occupational health disease?
3. What practice is among the Neelkanth miners regarding TB as an occupational health disease?
Methodology Study Site
This study was conducted at Neelkanth mine in Bwana Mkubwa area, this is an open pit mine in Ndola rural, copper belt province, Zambia. Neelkanth lime limited is located along Chilengwe Road, Southern extension of the Copper belt province, east of the T3 highway.
This mine mines lime, lime related products and electric cables. The population of the miners is over 500.
Target Population
The target population was the miners both female and male aged 18 years and above. This target population was chosen due to the fact that the miners are aged 18years and above.
Study Design
This was a cross sectional study that used a structured questionnaire to collect data from the study participants.
Sample Size
The following formula was used to determine the required sample size using the Statical programme in Epi Info version 7.
n
Sample size =
n
1 + Population size
P (1-P)
Where n = Z2
e2
|
Total Population Size |
118,464 |
|
Level of Confidence Measure |
1.96 (at 95% Confidence level) |
|
Margin of Error (MOE) |
5% |
|
Baseline levels of the indicators |
50% (as no estimates exist) |
|
Number of age/sex Estimates |
18 years and above |
|
Design effect (Deff) |
2.00 (as no information on previous surveys is available) |
|
Sample size |
384 |
n = Sample size, Z = critical value of the normal distribution at required confidence level, p = sample proportion, e = margin of error
Table I: showing sample size determination
Sampling Procedure
The survey sample is the set of survey participants selected from the larger survey population. The survey sampling determined how participants were selected and addressed the generalizability, certainty, and precision of results by defining who is included in the survey and how many people were needed.
In this study, the simple random sampling technique was used.
Inclusion And Exclusion Criteria
The inclusion criteria:
1. All male/female miners in specific job roles such as crushing, blasting or breaking (roles directly involved in dust) are eligible to participate in this study.
2. All miners willing to give consent The exclusion criteria:
1. Miners in administrative works (office based roles) will not be included in the study.
2. Miners not willing to give informed consent
Data Collection And Analysis
Three research assistants were recruited to collect the data. These were supervised by the Principal investigator. A paper questionnaire was used to elicit responses from the interviewees. This investigator and the research assistants went to the site of the mine and physically interview the participants. The investigators explained the objectives of the study to the miners. Written informed consent was obtained from each miner before being enrolled into the study. The questionnaire covering aspects of knowledge, attitude, and practice about TB as an occupational hazard were administered by the investigators to the study participants. The data was entered and analysed in Spss version 16.0. The collected data was analysed using the chi square test and multivariate logistic regression at 95% confidence level.
The questionnaire was divided into the following sections: interviewer name, information on knowledge, attitudes, and practices about TB. See Appendix 2.
Ethical Considerations
Ethical clearance for the study was obtained from the Tropical Diseases Research Centre (TDRC) ethics review committee and the National Health Research Ethics Committee (NHRC). Written Informed Consent was obtained from each study participant before being enrolled into the study. Confidentiality of information was observed.
Results
Demographic characteristics about study participants
A total of 384 participants were enrolled into the study but 27 questionnaires were incomplete and this resulted into the sample size being reduced to 357. Table 1 shows a summary of demographic characteristics about the study participants. Table 1 further shows that they were more male than female participants in the study with a ratio of 1.3:1. The mean age was calculated to be 32.9 (standard deviation [SD]: 7.4) years, with more than half of the participants aged between 18-40years. More than three quarters (82.9%) of the study participants were from households that had 1-7 occupants per household. More than half (65.5%) of the study participants had children between the ages 0-7. The majority of the participants (38.4%) had attained primary school education.
Table 1: Demographic Characteristics About Study Participants
|
Variable |
Frequency |
Percentage (%) |
|
Age groups 18-30 31-40 41-50 |
156 134 67 |
43.7 37.5 18.8 |
|
Sex Male Female |
202 155 |
56.6 43.4 |
|
Number of occupants per household 1-7 people 8-15 people |
296 61 |
82.9 17.1 |
|
Number of children per household 0-7 years 8-17 years |
234 123 |
65.5 34.5 |
|
Level of education attained None Primary Secondary Completed secondary/higher Refused to comment |
45 137 107 55 13 |
12.6 38.4 30.0 15.4 3.6 |
Table 2: Study Participants’ Response To Knowledge About Tb As An Occupational Health Disease
|
Question |
Frequency |
Percentage (%) |
|
|
What is TB? Can define Can’t define |
257 100 |
72.0 28.0 |
|
|
Do you know what causes TB? I know I don’t know |
208 149 |
58.3 41.7 |
|
|
What do you think causes TB? Smoking Exposure to silica dust Close to an infected person Working without PPE I do not know |
71 49 76 12 149 |
19.9 13.7 21.2 3.4 41.7 |
|
|
Among the listed risk factors below, what do you think causes TB? Crowed places Exposure to silica dust Close contact to the infected Working without PPE Poor ventilation at work |
34 153 93 39 38 |
9.5 42.9 26.1 10.9 10.6 |
|
|
Is TB preventable? Yes No |
199 158 |
55.7 44.3 |
|
|
Do you know how to protect yourself from TB? Yes No |
186 171 |
52.1 47.9 |
|
|
Do you know the sign and symptoms of TB? Yes No |
247 110 |
69.2 30.8 |
|
|
Can anyone get TB? Yes No |
252 105 |
70.6 29.4 |
|
|
Is TB curable? Yes No |
290 67 |
81.2 18.8 |
|
|
Is it important to finish TB treatment? Yes No |
249 108 |
69.7 30.3 |
|
|
Extent of knowing TB Extensively Little Nothing |
75 184 98 |
21.0 51.5 27.5 |
|
Table 2 shows study participants` response to knowledge about TB as an occupational health disease. The participants’ knowledge about TB etiology, prevention, protection, transmission and treatment as an occupational health disease is presented in Table 2. More than half (72%) of the participants’ had knowledge concerning definition, causes, risk factors and prevention (55.7%) of TB as an occupational health disease. The majority of the participants (70.6%) knew that anyone could get TB and 81.2% knew that TB can be cured. More than half (52.1%) knew how to protect themselves from TB and that it is important to finish TB treatment.
Table 3: Study Participants’ Response To Questions On Attitude About Tb As An Occupational Health Disease
|
Question |
Frequency |
Percentage (%) |
|
Is TB preventable in the mines? Yes No |
171 186 |
47.9 52.1 |
|
Do you think working in the mine increases the chance of contracting TB? Yes No |
191 166 |
53.5 46.5 |
|
Would you work closely with a colleague who has TB? Yes No |
81 276 |
22.7 77.3 |
|
Should a colleague with TB be allowed to come for work? Yes No |
122 235 |
34.2 65.8 |
|
What would be your reaction if your closest colleague suffers from TB? Not discriminate Discriminate |
204 153 |
57.1 42.9 |
|
Who do you think is responsible for preventing TB in the mines? Management Miners Both |
187 80 90 |
51.8 22.4 25.2 |
|
What should a miner do if fired from work because they have contracted TB? Report No report |
132 225 |
37.0 63.0 |
Table 3 shows study participants` response to questions on attitude about TB as an occupational health disease. Table 3 further shows that more than half (52.1%) of the participants said TB as an occupational health disease is not preventable in the mines and that working in the mine increases the risk of contracting TB. Two-thirds (77.3%) of the participants said they would not work closely with a colleague infected with TB and that a colleague with TB should not be allowed to come for work. More than half (57.1%) of the study participants would not discriminate a closest colleague who contracted TB and more than half (51.8%) of the participants said it is the responsibility of the mine management to prevent TB in the mine. More than half (63.0%) of the study participants said they would not report if they were fired because they contracted TB.
Table 4: Study Participants’ Response To Questions On Practice About Tb As An Occupational Health Disease
|
Question |
Frequency |
Percentage (%) |
|
Do you know how to protect yourself from TB? I know I don’t know |
186 171 |
52.1 47.9 |
|
Do you wear PPE? Yes No |
247 110 |
69.2 30.8 |
|
Do you go for regular chest examinations? Yes No |
131 226 |
36.7 63.3 |
|
Why do you do regular chest checkups? Important Not important |
229 128 |
63.4 35.5 |
|
Were you screened for TB at the time of being employed as a miner? Yes No |
198 159 |
54.8 44.0 |
Table 4 shows study participants` response to questions on practice about TB as an occupational health disease. Table 4 further shows that more than half (52.1%) of the study participants knew how to protect themselves from TB as an occupational health disease and almost two-thirds (69.2%) of the participant’s wear PPE as they work. More than half (63.3%) of the study participants do not go for regular chest examinations and more than half (63.4%) of the participants know that it is important to go for regular chest checkups. More than half (54.8%) of the study participants were screened for TB at the time of employment. The difference of practice levels about TB as an occupational health hazard and sex (male/female) was good (52.1%).
Table 5: Association Between Study Participants’ Demographic Characteristics And Awareness Of Tb As An Occupational Health Disease
|
|
Awareness of TB as an Occupational health disease |
|||
|
|
Yes |
No |
|
|
|
Variable |
n(%) |
n(%) |
P value |
|
|
Age groups |
18-30yrs 31-40yrs 41-50yrs |
30(49.2) 28(45.9) 3(4.9) |
94(31.8) 128(43.2) 74(25.0) |
0.001 |
|
Sex |
Male Female |
22(36.1) 39(63.9) |
180(60.8) 116(39.2) |
<0.001 |
|
House groups |
1-7people 8-15people |
34(55.7) 27(44.3) |
170(57.4) 126(42.6) |
0.808 |
|
Children groups |
0-7yrs 8-17yrs |
56(91.8) 5(8.2) |
266(89.9) 30(10.1) |
0.643 |
|
Level of education none Primary Secondary Completed secondary/higher Refused to comment |
6(9.8) 24(39.3) 22(36.1) 9(14.8) 0(0.0) |
39(13.2) 113(38.2) 85(28.7) 46(15.5) 13(4.4) |
0.396 |
|
Table 5 shows the association between demographic characteristics of study participants and awareness of TB as an occupational health disease. There was a statistically significant association between participants age groups and awareness of TB as an occupational health disease with (P<0.05). There was also a positive significant association between sex and TB as an occupational health disease with (P<0.05).
Table 6: Association Between Knowledge Levels And Tb As An Occupational Health Disease
|
|
TB As an occupational health disease |
|||
|
|
Yes |
No |
|
|
|
Variable |
n(%) |
n(%) |
P value |
|
|
What is TB? |
Yes No |
55(90.2) 6(9.8) |
202(68.2) 94(31.8) |
0.001 |
|
What Causes TB? |
Yes No |
45(73.8) 16(26.2) |
163(55.1) 133(44.9) |
0.007 |
|
Risks factors of TB? Crowed places Exposure to dust Close to infected person Without PPE Poor ventilation |
9(14.8) 30(49.2) 10(16.4) 7(11.5) 5(8.2) |
25(8.4) 123(41.6) 83(28.0) 32(10.8) 33(11.1) |
0.209 |
|
|
Is TB Preventable? |
Yes No |
39(63.9) 22(36.1) |
160(54.1) 136(45.9) |
0.157 |
|
Do you know how to Protect yourself? Yes No |
37(60.7) 24(39.3) |
149(50.3) 147(49.7) |
0.142 |
|
|
Do you know sign and symptoms of TB? Yes No |
47(77.0) 14(23.0) |
200(67.6) 96(32.4) |
0.144 |
|
|
Can anyone Get TB? |
Yes No |
53(86.9) 8(13.1) |
199(67.2) 97(32.8) |
0.002 |
|
Is TB curable? |
Yes No |
59(96.7) 2(3.3) |
231(78.0) 65(22.0) |
0.001 |
|
Finishing TB treatment important? Yes No |
45(73.8) 16(26.2) |
204(68.9) 92(31.1) |
0.453 |
|
Table 6 shows the association between knowledge levels and TB as an occupational health disease. Table 6 also shows that, there was a positive significant association between what is TB and TB as an occupational health disease with a (P<0.05). Table 6 further shows that there was a positive significant association between what causes TB and TB as an occupational health disease with a (P<0.05). There was also a positive significant association between can anyone get TB and TB as an occupational health disease with a (P<0.05). Lastly, there was a positive association between is TB curable and TB as an occupational health disease with (P<0.05).
Table 7: Association Between Attitude Levels And Tb As An Occupational Health Disease
|
|
TB As An Occupational Health Disease |
||
|
|
Yes |
No |
|
|
Variable |
N (%) |
N (%) |
P value |
|
Is TB Preventable in mines? Yes No |
46(75.4) 15(24.6) |
125(42.2) 171(57.8) |
<0.001 |
|
Working in the mine increases risk of Contracting TB? Yes No |
31(50.8) 30(49.2) |
160(54.1) 136(45.9) |
0.645 |
|
Would you work closely with colleague who has TB? Yes No |
28(45.9) 33(54.1) |
53(17.9) 243(82.1) |
<0.001 |
|
Should infected workers be allowed to work? Yes No |
33(54.1) 28(45.9) |
89(30.1) 207(69.9) |
<0.001 |
|
Would you discrimate infected colleagues? Yes No |
36(59.0) 25(41.0) |
168(56.8) 128(43.2) |
0.745 |
|
Who is Responsible for prevention of TB in mines? Management Miners Both |
43(70.5) 5(8.2) 13(21.3) |
144(48.6) 75(25.3) 77(26.0) |
0.003 |
|
If fired due to TB, would you report? Yes No |
30(49.2) 31(50.8) |
102(34.5) 194(65.5) |
<0.001 |
Table 7 shows the association between attitude levels and TB as an occupational health disease. Table 7 shows that there was a positive significant association between is TB preventable in mines and TB as an occupational health disease with a (P<0.05). There was a positive significant association between would you work closely with colleague who has TB and TB as an occupational health disease with a (P<0.05). Should infected workers be allowed to work and TB as an occupational health disease showed a positive significant association with a (P<0.05). There was a positive significant association between who is responsible for prevention of TB in mines and TB as an occupational health disease with a (P<0.05). Lastly, there was a positive significant association between if fired due to TB would you report and TB as an occupational health disease with a (P<0.05).
Table 8: Association Between Practice Levels And Tb As An Occupational Health Disease
|
|
TB As An Occupational Health Disease |
||
|
|
Yes |
No |
|
|
Variable |
N (%) |
N (%) |
P value |
|
Do you know how to protect yourself from TB? Yes No |
37(60.7) 24(39.3) |
149(50.3) 147(49.7) |
0.142 |
|
Do you wear PPE? Yes No |
47(77.0) 14(23.0) |
200(67.6) 96(32.4) |
0.144 |
|
Do you go for chest exam? Yes No |
38(62.3) 23(37.7) |
93(31.4) 203(68.6) |
<0.001 |
|
Are checkups important? Yes No |
21(34.4) 40(65.6) |
119(40.2) 177(59.8) |
0.400 |
|
Were you Screened? Yes No |
24(39.3) 37(60.7) |
114(38.5) 182(61.5) |
0.903 |
Table 8 shows the association between practice levels and TB as an occupational health disease. The table shows that, there was a positive significant association between the study participants that do go for regular chest exams and TB as an occupational health disease with a (P<0.05). The table also shows that there was no significant association between participants who did not know how to protect themselves from TB and TB as an occupational health disease with a (P>0.05).
Table 9: Multivariate Logistic Regression Output And Association To Tb As An Occupational Health Disease
|
Question |
AOR |
95% CI |
|
What is TB? Yes No |
1.84 1 |
1.17,2.91 |
|
Is TB Preventable in the mines? Yes No |
1.66 1 |
1.18,2.32 |
|
Do you wear PPE? Yes No |
1.71 2 |
1.25,2.34 |
|
Do you go for regular chest exam? Yes No |
1.55 2 |
1.13,2.11 |
Table 9 shows multivariate logistic regression output and association to TB as an occupational health disease. The table shows results of multivariate logistic regression analysis of four variables that had an independent and statistically significant association to TB as an occupational health disease. Comparing the participants that could define TB as an occupational health disease to those that could not, those that could define were 1.84 times (CI95: 1.17, 2.91) more likely to define. Likewise, participants were 1.66 times (CI95: 1.18, 2.32) more likely to know preventive measure of TB as an occupational health disease compared to those who did not know. Participants who wear PPE wear more than twice (CI95: 1.25, 2.34) likely to protect themselves from TB compared to their counterparts who did not. Participants who did not go for regular chest examination were twice (CI95: 1.13, 2.11) more likely to have TB compared to the counter parts who did not.
Discussion
This study assessed the knowledge, attitude and practices about pulmonary Tuberculosis as an occupational health disease at Neelkanth mine in Ndola, Zambia. In terms of knowledge, the majority (72%) of the study participants knew what TB is and this is below the findings in a similar study done in the mines on the Copperbelt and North Western provinces of Zambia
[101] in which a 94% knowledge level was recorded. This difference shows that there is need for TB education among Neelkanth miners.
It was revealed in this study that more than half (53.5%) of the respondents indicated that working in the mine increases the risk of one contracting TB due to the fact that the mine provides little to no PPE and also the miners themselves do take off aspirators due to the fact that the environment they work in has poor air circulation and ventilation, this is consist with other studies that were done in South Africa [73]. Our research also revealed that two-thirds of miners stigmatise against TB infected colleagues and would not want to work closely with them. This is consistent in the research that was conducted in other Zambian mines [93] in which it was found that the majority of miners would not disclose to their supervisor over a TB diagnosis for fear of job loss.
Our study also showed that more than half of the respondents had not suffered from TB since joining this mine, this is consistent with studies done in other mines in Zambia [101] in which it was found that less than half of the respondents had tested positive for TB [101]. It was also observed that more than half of the miners at this mine did not go for regular chest examination this may be due to the fact that the mine does not cover the costs of regular chest examinations and this does not help the miners as exposure to silica dust has been associated with TB. Silica-related TB is a serious condition since the risk of TB is life-long even after exposure ceases [96].
There was limited data and research in Zambia to aid in fully determining the burden of TB in the mines and the risk factors associated with it. However, the few studies and data available show that TB is a significant health problem among miners in Zambia [93].
The implications of this study findings include, the fact that this study was only done at one mine, it may not be a representation of all Zambian mines and therefore, these findings cannot be generalised to include all mines in Zambia. Although, the research assistants were trained to assist in data collection, we cannot rule out the possibility of social desirability bias. Due to the literacy levels among our study participants, the research assistants had to read and explain in a local language to some of the participants for them to understand what the question was asking, in spite of the merits, one of the disadvantages is the possibility of having the interviewer effects leading to the interviewer bias.
Study Limitations
The study design provided reliable, valid information with a few limitations such as, miners were not coming out freely in the open to admit that they have TB or have had TB for fear of being discriminated, stigmatised and dismissed from work as the mines do not want to be liable for their health. Some Participants were not finishing answering the whole questionnaire and this affected the overall sample size and reduced it from 384 to 357.
Recommendation
Based on the findings of this research, it is cardinal for mines to consider TB education workshops once in a while for its miners. Also TB flyers and posters can be hanged within the mine premise to raise awareness.
There is also need for government through the ministry of mines and mineral development to strengthen laws against mines that are operating with miners who are not provided with full proper PPE, pre-chest examinations and regular chest examinations. This will greatly help in the reduction of TB in the mining sector.
The occupational Health and Safety Institute is the institute responsible for ensuring that all workers and former workers in industries including miners and former miners regularly and routinely screened for occupation health diseases including TB. It is therefore, very cardinal for this branch of government to run effectively and without bias in ensuring the miners are properly screened for occupational health disease (TB) and recommend all miners found to be sick to worker’s compensation union. We strongly recommend that the worker’s compensation union advocate for miners who have completed TB treatment and are fit to work to come back to their respective mine. This will encourage other miners to routinely go for check-ups and not to be afraid of the outcome should they be found with PTB or silica- related TB.
Conclusion
The study revealed that the knowledge levels were good, however, this was not effectively seen in their attitude and practice levels as these were lower than the knowledge level. This study also revealed that there is a significant association between knowledge, attitude, practice and TB as an occupational health disease. These findings highlight the need for TB education among miners.
List of Abbreviations
COPD chronic obstructive pulmonary disease
HIV Human immune deficiency virus
MTB Mycobacterium tuberculosis
PTB Pulmonary Tuberculosis
SDG Sustainable development goals
SSA Sub Saharan Africa
TB Tuberculosis
TDRC Tropical disease research centre
UN United Nations
WHO World Health Organisation