
Ahmad Reza Rahnemoon
Department of hematology, Iran University of Medical Sciences, Tehran, Iran.
*Corresponding Author: Ahmad Reza Rahnemoon, Department of hematology, Iran University of Medical Sciences, Tehran, Iran.
Received: December 07, 2022
Accepted: February 10, 2023
Published: March 06, 2023
Citation: Ahmad Reza Rahnemoon. (2023) “Can Genetic Abnormalities Makeup Leukemia (e.g., Chronic Myeloid Leukemia) Only?”, J Oncology and Cancer Screening, 5(1); DOI: http;//doi.org/03.2023/1.1065.
Copyright: © 2023 Ahmad Reza Rahnemoon. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Niche have an essential role for self- renewal and differentiation of HSCs in vivo. So in the hematopoietic microenvironment, the cells generate functional hematopoietic stem cells and other progenitors that go to differentiation and maturation of cells which means these are as the important key points in normal state and hematopoietic malignant disorders. Hence, understanding of stem-ness properties and the important cells such as HSC, progenitor and precursor cells which we’ll be able to the topic perceiving as 1) the basic power 2) the best engine, for drive to sequential rounds of leukemia development.
niche; hematopoietic stem cell; leukemia; genetic aberrations; bone marrow; blood cell morphology
Introduction:
Leukemia is the consequence of step-wise genetic alterations that confer both proliferative and survival advantage, as well as self-renewal capacity to the malignant cells. Leukemia stem cells (LSCs) possess several key properties of normal cells including self -renewal, unlimited proliferative potential, infrequent or slow replication. LSCs infiltrate the bone marrow and interfere with the normal HSC microenvironment hemostasis. Thus, the major difference between leukemia growth and normal tissue renewal is that whereas normal transit amplifying cells usually differentiate and die at various levels of differentiation, the leukemia transit-amplifying cells can go to differentiate abanormally and instead, accumulate, resulting in leukemia growth. [1-4].
The cyto-morphologic characteristics of blasts are varied but are usually sufficient to suggest a blastic or neoplastic process for which phenotyping can confirm and further characterize the process. It is mentionable that in some cases, the blasts exhibit significant morphological variation. Such nuclear outlines and less homogeneous chromatin. Nuclei are variable but frequenly prominent, and sometimes multiple. The cytoplasm is more abundant but still pale blue. But I try to discuss on a) the important role of niche b) more attention to bone marrow and peripheral blood smears (or blood cells morphology).
Discussion:
In chronic myeloid leukemia (CML), sustained by a range of biological characteristics that enable their long-term survival, and accumulation of myeloid cells that differentiate in normal and abnormal clones, which can change to acute lymphoblastic leukemia(ALL) in accelerated phase possibly. As we know in the microenvironment and stem cell niche unit, HSC self- renewal, control in balance between HSCs self- renewal, their differentiation and maturation can be important as well, particularly in CML can emboss, because of in CML , we have three involved lineages includes neutrophilic series, eosinophilic and/or basophilic lineages that create the special morphology which be as a key point at the differential diagnosis. In fact, loss of normal hematopoiesis is an event occurring during the development of most leukemias. Some researchers stated that leukemic hematopoiesis turns the endosteal BM niche into a leukemic niche, which promotes LSC function and impairs the maintenance of normal HSCs that may be to contribute to malignant cases like myelofibrosis development. These actions expand our understanding of the effects of leukemic hematopoiesis on the BM microenvironment and the contribution of the endosteal BM niche to myeloproliferative neoplasms pathogenesis and similar cases too. Also leukemia and its development alters the normal activity of MSCs and their osteoblastic lineage cell(OBC) derivatives, leading to a major remodeling of the endosteal BM niche, which mainly affects normal HSCs, with minimal effects on transformed LSCs. The main fact that LSC maintenance is unaffected by the remodeled OBCs could be, in large part, due to their different requirement in adhesion molecules for homing and retention in the BM compared to normal HSCs. [1-2,5-9]
In addition we can see the other abnormality in this leukemia; while in the initial step, one aberrant gene is possibly involved such as BCR-ABL210 in the case. I remind that some stem cells are involved in CML patients including pluripotent stem cell, CFU-G(colony forming unit- granulocyte,), CFU-eosinophil, CFU-basophil and CFU-megakaryocyte. In CML early proliferative progenitors have been shown to be defective in their ability to bind to stromal monolayers. In this regard, there is an intrinsic abnormality in the capacity of primitive hematopoietic progenitors in CML to interact with stromal elements. Moreover, CML long term hematopoietic stem cell (CML LTHSC) reduced homing and retention in bone marrow, resulting from increased G-CSF production by leukemic cells. Altered cytokine expression in CML bone marrow was associated with selective impairment of normal LTHSC growth and a growth advantage to CML LTHSC. Furthermore, In CML both leukemic and quiescent normal hematopoietic stem cells preferentially reside in the osteoblastic niche . In fact, the structure of bone marrow niche must intact, and after the impair of hematopoiesis particularly in malignant hematopoietic diseases, BM should change. So my question is, what happen after bone marrow transplantation (BMT) in CP-CML patients? In response, our purpose of BMT or HSC transplantation(HSCT) is repair the structure of BM by HSCs maintenance chiefly and other cells of BM niche at keeping in normal state and good condition basically.[1-3,10-14]
Myeloid hyperplasia in CML:
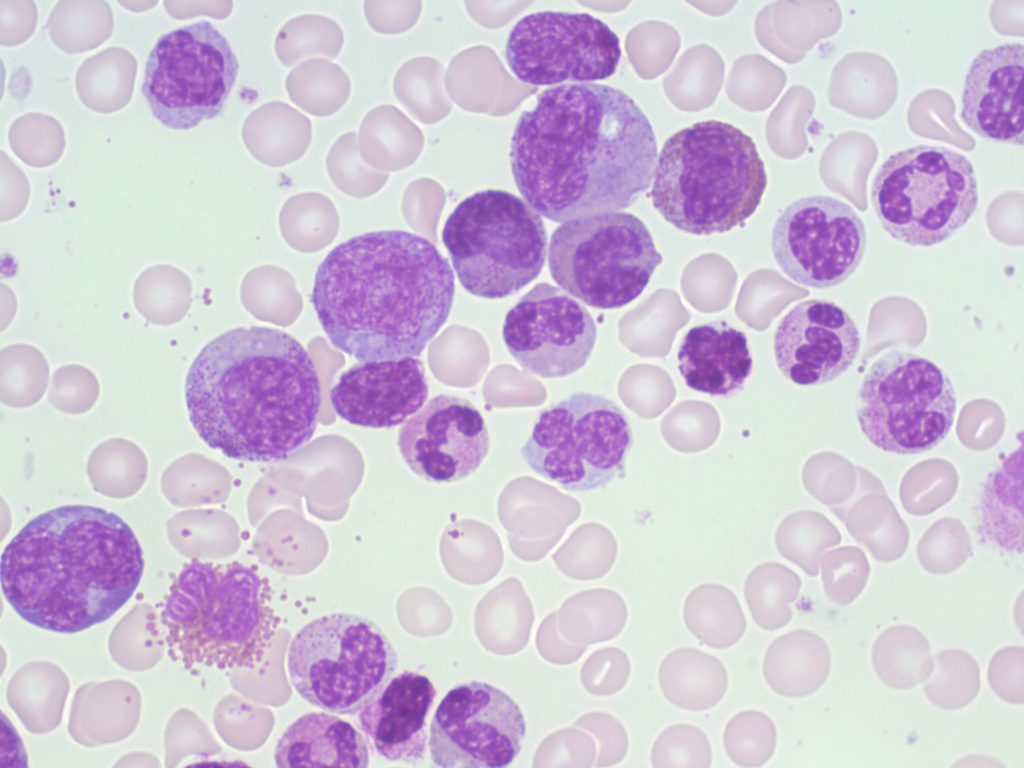
Figure 1: The peripheral blood shows mild anemia and leukocytosis with left shift, increases at neutrophils series in maturation various stages particularly, in myelocytes and mature neutrophils, in addition eosinophilia is common and usually platelet count is elevated.( Alberta University) [6,28]
On the other hand, genomic profiling transformed our understanding of the genetic basis of leukemia particularly in ALL, which is a malignant clonal proliferation of lymphoid progenitor cells. , if we look to childhood BM niche, we can find the ability and power of childhood BM in re-creativity and rebuild soon in disrupted BM particularly in progenitors, precursors , and the cells like blasts and maturation cells as well and the other cells in BM niche additionally and so we can understand the important role of HSCs in childhood disease . Told all, In my discussion I want to explain the complexity of BM niche cells, and increasingly in the essential role of malignant stem cell with self-renewing toward capable initiating and maintaining of leukemia as well.
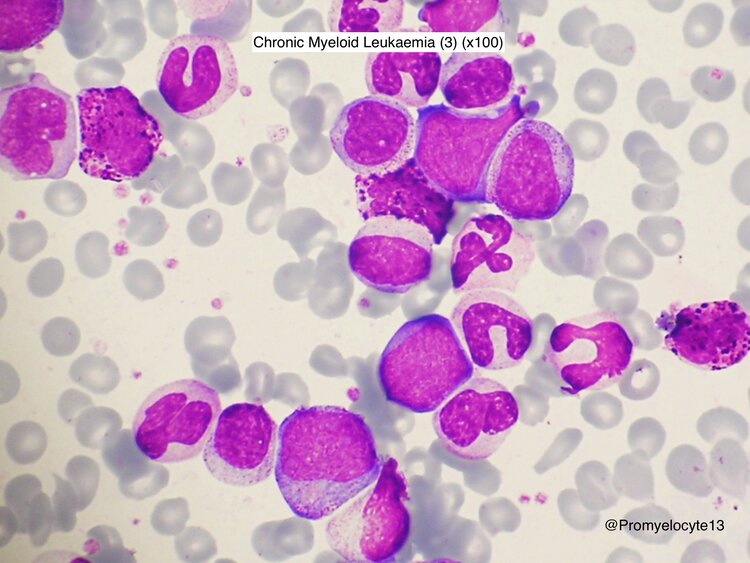
Figure 2: CML, chronic typical phase .Marked proliferation of the cells in the myeloid series are presented, including the blasts, myelocytes, etc. Also look at the basophils which stated in the demonstration two series of the cells including neutrophilic and basophilic series are involved. [27-28]
Moreover, we know cytogenetic and molecular testing in leukemia is integral for the diagnosis. Also, conventional chromosome analysis is a basic way for diagnosis and treatment. In addition in this way evaluation of disease progression is important and so it is the only method that can identify the presence of clonal evolution, particularly in accelerated and relapse phase in the disease. Also conventional cytogenetic can detect chromosomal abnormality associated with its advanced phase . For fusion genes studies FISH is a more sensitive test in the advantage of routinely interrogating 50 to 200 metaphase or interphase cells .However, one of the most sensitive test is RT-PCR in molecular fusion gene study and MRD assay as well. It is notice-able, the value of translocation rates in interphase and metaphase nuclei in monitoring leukemia is at the time of diagnosis and after treatment as well. [6,13-18]
In connection with the matter, we know some researchers stated several rearrangements like BCR/ABL which detected in normal persons. Somebody reported that BCR/ABL transcripts found in healthy individuals differ from leukemic individuals but why? Because of they believed that produce truncated protein which unable to promote proliferation and differentiation that actually it can`t be accepted (as a true BCR/ABL). Is it right? Also, it is noticeable that in a case , the patient with CML diagnosed at the pregnancy beginning which was treated with leukapheresis and so in practice no transcripts were detected in the cord blood or in the peripheral blood of newborn at birth, at one month and after three months of age, while her mother in pregnancy and after delivery was with 90% BCR/ABL positive cells in her blood. So this subject is absolutely big enough to be noticed. Furthermore, Ph chromosome (BCR/ABL210) is usually detected in CML, but what about other forms of BCR/ABL like BCR/ABL190 and BCR/ABL230 which can be in CML patients as well. P190 is associated with Ph positive ALL in 70% of cases and infrequent in CML cases. A P230 BCR/ABL isoform is found in CML patients rarely. Noticeably, some cases associated in resembling with chronic neutrophilic leukemia (CNL) and may be have a lower rate of acute transformation like AML. In this regard, CNL is characterized by leukocytosis, a predominant neutrophilia with normal morphology and so neutrophil precursors like meta-myelocyte, myelocyte and promyelocyte is less than 10% totally which can differentiate CNL from typical CML and atypical CML(aCML) as well. [13-16,19-21,25]
The other aberration is ETV6/ABL which can be detected in CML, and some researchers stated a CML case report with JAK2V617F mutation with Ph+ in same time, and others reported several CML cases were with the coexistence of JAK2V617F and Ph+ as well. On the other part, In a early chronic phase (ECP)of a CML patient was with polyclonal CD34+ cells, ph- and BCR/ABL mRNA- could only be found in the first peripheral blood collection and in late chronic phase and AP, the cells were mostly Ph+, BCR/ABL mRNA+ and clonal as well. So we know, CML is a kind of leukemia creating the fusion oncogene BCR/ABL210, but why one disease and some different rearrangements or fusion genes? And secondly, how is it possible? Does the identification of mRNA BCR/ABL not necessarily indicate this leukemic disease directly? Is it right? If yes, how is this possible? On the other hand, the interactions between BCR/ABL1 and secondary messengers such as GRB2, GAB2, CRK1 lead to apoptosis inhibitor and increased proliferation and differentiation through the PI3K/AKT, JAK/STAT and RAS/RAF/MEK pathways activation. BCR/ABL also leads to genomic instability? What does it mean exactly? Perhaps, it means some different interpretations including 1- It can produce truncated proteins which are unable to promote proliferation of the cells. 2- The fusion gene does not seem to be predictive of a developing malignancy, but rather they are possibly to be indicative of transient genomic instability. So, what does this mean ? Which one is right in genomic instability? Secondly, are the above interpretations opposite with each other? On the other hand, what is responsible for some fusion gene which its generation is happened in normal fetal hematopoiesis? In response: a) Presumably, non-functional fusions in non-stem cells which occur at an even higher rate. Is it right? If yes, why some of these fusion genes can change to malignant diseases after short or long time? b) These are normal developmental errors of DNA maintenance reflecting the embryo complexity as well as fetal tissue engineering in which point oxidative stress ,DNA damage and cell death are ubiquitous. Is it ambiguous? Isn`t it? Also, is it as a general concept and so it is not the satisfied response in this matter. [13-16,19-24]
Now then, what about BCR/ABL negative CML? As we know, aCML is a rare hematologic malignancy with BCR/ABL negative including the presence of leukocytosis with circulating neutrophil precursors, minimal basophils, BM hyper-cellularity and granulocytic proliferation and dysplasia. In addition, blood and BM blast count should be less than 20%. Also, aCML can be associated with some mutations such as CSF3R, SETBP1, JAK2V617F. In the patients, KRAS or NRAS mutations may also be common. Some genetic abnormalities which are similar and it is important in the leukemic differential diagnosis as follows: a) CSF3R and SETBP1 genes have been found in CNL b) CSF3R can be mutated in AML and T-ALL c) SETBP1 is found in chronic myelomonocytic leukemia (CMML) d) CSF3R is important gene in congenital neutropenia. Here we have different cases with the same genetic aberrations, So why ? What does it mean? [3,10,13,15,20-21]

Figure 3: CML in progression. The patients are usually diagnosed in chronic phase that will progress through the accelerated phase to blast crisis (including AML and sometimes ALL) if untreated which resulting in increased proliferation of blasts and other immature cells and decreased apoptosis as well. [4-6,26]
In the end:
Differential diagnostic considerations based on the cytomorphological and histological features of blasts in the peripheral blood and marrow depend in part on the patient’s age. Supposedly, in pediatric patients with high peripheral blood counts, some infectious disease like pertussis must be considered. In both children and adults, the differential also includes acute myeloid leukemia (AML), bi-phenotypic leukemia and CML presenting in lymphoid blast phase. For example, although mixed lineage leukemia is rare, most cases bear the t [9;22] lesion. These cases with [9;22] make up less than 1% of acute leukemias which recognized as a separate entity of hematopoietic neoplasms. Also, in BP-CML, 70% of cases are with the blasts with myeloid lineage. In addition, these cases may express antigens associated with granulocytic, monocytic, megakaryocytic or erythroid differentiation as well. It is noticeable that aberrant expression of myeloid antigens in lymphoid BP-CML is quite common too. So in all cases, immunophenotyping by flow cytometry or by immunohistochemistry can be resolved in the case of diagnostic dilemma and should be recommended. Surely, the interpretation of flow-cytometric data have the phenotype exact knowledge of diverse normal cell populations which can be one of the best tool as a major role in final diagnosis and in determination of myeloid and lymphoid lineages at immunophenotyping category as well as acute and chronic leukemias classification additionally. It is noticeable that the new knowledge has increased the diagnosis accuracy and allowed the identification of very small populations and subpopulations. Also, it can be very useful in identifying at the specific differences in normal cells and leukemic populations in residual disease monitoring as well. Hence, the exact hematological neoplasms classification is critical which is including a) for the clinician`s choice of therapy b) for overall prognosis. [22-26,29]