Oncology and Cancer Screening
OPEN ACCESS | Volume 7 - Issue 1 - 2026
ISSN No: 2994-8746 | Journal DOI: 10.61148/2994-8746/JOCS
Paolo Lissoni 1, Giorgio Porro 1, Franco Rovelli 1, Giusy Messina 1, Giuseppe Di Fede 1, Daniel Pedro Cardinali 2*.
1Institute of Biological Medicine, Milan, Italy
*2Pontificia Universidad Catolica, Buenos Aires, Argentina.
*Corresponding author: Daniel Pedro Cardinali, Pontificia Universidad Catolica, Buenos Aires, Argentina.
Received: April 25, 2022
Accepted: May 10, 2022
Published: May 17, 2022
Citation: Paolo Lissoni, Giorgio Porro, Franco Rovelli, Giusy Messina, Giuseppe Di Fede, Daniel Pedro Cardinali. (2022) “A Phase 2 Study with Chemotherapy in Association with Melatonin Plus Angiotensin 1-7 in Advanced Cancer Patients”, J Oncology and Cancer Screening, 4(1); DOI: http;//doi.org/005.2022/1.1052.
Copyright: © 2022 Daniel Pedro Cardinali. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The recent advances in the knowledge of tumor biology have shown that the efficacy of chemotherapy depends not only on its direct effects on cancer cell proliferation, but also on its modulatory action on the cytokine network, which may either improve or furtherly suppress the antitumor immunity. Therefore, it is important to evaluate the changes in immune status under cancer chemotherapy, at least by analyzing the variations of lymphocyte-to-monocyte ratio (LMR), whose decline has been shown to predict a poor prognosis and a lack of response to chemotherapy itself. It has already been demonstrated that the simple association with the pineal immunomodulating hormone melatonin (MLT) may enhance the efficacy of chemotherapy and the duration of response. Recently, it has been shown that another endogenous molecule may exert antitumor activity, the angiotensin 1-7(Ang 1-7), which is the enzymatic product of ACE2. On these bases, a preliminary study was planned to evaluate the influence of a concomitant oral administration of both MLT (100 mg/day in the evening) and Ang1-7 (0.5 mg twice/day) on chemotherapy in a group of advanced cancer patients.The study included 14 consecutive advanced cancer patients affected by different tumour histotypes, who had been patients already pre-treated with at least a chemotherapeutic line. No patient progressed on chemotherapy, since 9/14 (64%) patients achieved an objective tumour regression and the other 5 obtained a stable disease. Moreover, most patients experienced relief of asthenia and anxiety, and an improvement in mood. These preliminary results would suggest that a concomitant administration of the endogenous antitumor molecules MLT and Ang 1-7 may enhance the efficacy of cancer chemotherapy with respect to the expected one in relation to tumour histotype and disease extension, even though further randomized studies will be needed to confirm these preliminary promising results
Introduction:
Today, it is known that the efficacy of cancer chemotherapy depends not only on the direct cytotoxic action of the various chemotherapeutic agents, but also on their modulatory effects on host immune status and on the angiogenic processes [1]. It is a common opinion that cancer chemotherapy may constantly exert only immunosuppressive effects. On the contrary, cancer chemotherapy tends constantly to play an immunosuppressive activity only on the antibacterial immunity because of its inhibitory action on neutrophil production, whereas its effects on the anticancer immunity, which is mainly mediated by lymphocytes [2,3], may be either inhibitory or stimulatory [1], and the action of chemotherapy on the antitumor immunity would be responsible for the clinical response to chemotherapy itself, either in terms of objective tumor regression, or particularly on the duration of the response. Most T lymphocyte subsets, including Th1 and cytotoxic T lymphocytes, play an antitumor action, whereas both regulatory T lymphocytes (T reg) [4] and Th17 lymphocytes [5] exert a pro-tumoral action, respectively by inhibiting the TH1-and cytotoxic T cell-mediated anticancer immunity and directly stimulating cancer cell proliferation. On the contrary, monocytes constantly play a major pro-tumoral activity, since monocyte count has been shown to positively correlate to tumor infiltration by macrophages, which has appeared to stimulate cancer growth and dissemination [6]. Therefore, despite the great complexity in the mechanisms involved in the anticancer immune, from a clinical point of view the antitumor immunity of each cancer patient may be simply established in relation to lymphocyte-to-monocyte ratio (LMR), since the evidence of an abnormally low LMR value is appeared to predict a low response to chemotherapy and a lower survival time [7,8]. Therefore, the manipulation of the effects of chemotherapy on the anticancer immunity could enhance the efficacy of cancer chemotherapy itself, mainly in terms of response duration and disease stabilization. In experimental conditions, the pineal hormone melatonin (MLT) has been shown to abrogate chemotherapy-induced immunosuppression, with a following increased efficacy of chemotherapy itself [9]. At present, several natural agents and have been proposed and are commonly used in the complementary therapy of cancer to integrate in some way the action of chemotherapy, but at present only the association between chemotherapy and the pineal indole MLT has appeared to enhance the efficacy of cancer chemotherapy and reduce its biological toxicity in humans [10,11]. Therefore, the aim of the complementary medicine would consist of furtherly increase the already interesting results achieved by the only MLT. In addition to the fundamental physiological anticancer anti-inflammatory role of the pineal gland through the production of MLT and other less known hormones, it has been demonstrated the existence of another fundamental natural anticancer anti-inflammatory system, represented by ACE2-angiotensin1-7 (Ang 1-7) axis, whose biological activity is opposite to that played by ACE-angiotensin II (Ang II) axis [12,13]. In fact, Ang II has appeared to play pro-tumoral, angiogenic, inflammatory and thrombotic effects, whereas Ang 1-7 exerts anti-tumor, anti-inflammatory and anti-thrombotic activities. Both MLT [14] and Ang 1-7 [15] play an anti-cancer activity through several mechanisms, including a direct inhibitory cytotoxic action on cancer cell proliferation, an anti-angiogenic activity and stimulation of the anticancer immunity, due to a stimulation of lymphocyte proliferation in association with a concomitant inhibitory action on monocyte-macrophage system [9,15]. Moreover, as well as MLT [10,11], preliminary clinical studies have shown that Ang 1-7 may also improve the tolerability of cancer chemotherapy [16,17]. On these bases, a preliminary phase II study has been performed to evaluate the efficacy of a chemotherapeutic neuroendocrine regimen consisting of chemotherapy plus MLT and Ang 1-7 in advanced cancer patients, and to obtain some preliminary data concerning its efficacy with respect to that previously described with chemotherapy in association with the only MLT [11].
Patients and Methods:
The study included 14 consecutive advanced cancer patients (M/F: 9/14; median age: 58 years, range 32-72). Eligibility criteria were, as follows: histologically proven solid neoplasms, measurable lesions, locally advanced or metastatic disease, at least one previous chemotherapeutic line, no double tumour, and no concomitant chronic therapy with corticosteroids or mu-opioids because of their immunosuppressive action. After the approval of the Ethical Committee, the clinical protocol was explained to each patient, and written consent was obtained. Tumour histotypes were, as follows: glioblastoma: 4; gynecologic tumours: 3 (ovarian cancer: 1; endometrial adenocarcinoma:1; uterine cervix carcinoma: 1), breast cancer: 2 (triple negative breast cancer: 1), rectal cancer: 1; pancreatic adenocarcinoma: 1; soft tissue sarcoma: 1; osteosarcoma: 1; lung adenocarcinoma: 1. Distant organ dominant metastases were present in 10 patients (nodes: 1; bone: 1; lung: 1; liver: 2; peritoneum: 2; brain 3), while the other four patients had a locally advanced brain glioblastoma. According to its light/dark circadian rhythm [18], MLT was given orally at 100 mg/day, generally 30 minutes prior to sleep. Ang 1-7 was also given orally in gastro-protected capsules at a dose of 0.5 mg twice/day (8 AM and 8 PM). We decided to use Ang 1-7 at low doses with respect to those previously described in the few studies reported in the literature [16,17] because of the stimulatory action of MLT on ACE2 expression, with a consequent promoting effect on the endogenous production of Ang 1-7 itself (20). The treatment was continued without interruption, including during the day of the administration of chemotherapy. The clinical response was evaluated according to WHO criteria by repeating the radiological examinations, including CT scan, NMR and PET, at 3-month intervals. The immune biomarker chosen to monitor host immune response was LMR itself, because of its demonstrated prognostic significance [7,8]. Normal values of LMR observed in our laboratory (95% confidence limits) were higher than 2.1. In patients with abnormally low LMR values, the onset of chemotherapy was preceded by a treatment with MLT plus Ang 1-7alone to improve the immune status of patients. LMR values were determined at 15-day intervals. Data were reported as mean +/- SE, and statistically analyzed by the chi-square test, the Student’s t-test and the analysis of variance, as appropriate.
Results:
The characteristics of patients and their clinical response to chemotherapy are reported in Table 1. Surprisingly, no patient had a progressive disease (PD) in response to cancer chemotherapy. On the contrary, a complete response (CR) was achieved in 4/14 (28%) (glioblastoma: 2; uterine cervix cancer: 1; ovarian cancer: 1). A partial response (PR) was obtained in other 5/14 (36%) (pancreatic cancer:1; lung adenocarcinoma: 1; glioblastoma; rectal cancer: 1; endometrial cancer: 1). Therefore, an objective tumour regression (CR +PR) was achieved in 9/14 (64%). The remaining five patients had a stable disease (SD). The median duration of response was 10+ months (range 5 -16+ months). From an immune point of view, abnormally low values of LMR were found in 7/14 (50%) patients prior to therapy, which was due to low lymphocyte count and/or enhanced monocyte number. LMR increase on therapy in all patients, with normalization of its values in 6/7 (86%) patients with abnormally low values of LMR before the study. Moreover, the mean increase in LMR values presented after at least three cycles of chemotherapy was significantly higher in patients who achieved an objective tumour regression than in those who had a disease stabilization (1.2 +/- 0.3 vs 0.3 +/- 0.1, P< 0.05). No MLT plus Ang 1-7-related specific toxicity was noted.On the contrary, most patients referred an improvement in mood and a relief of anxiety and asthenia. Moreover, an evident increase in the diuresis was referred by 10/14 (71%) patients. Finally, the safety of chemotherapy was referred by the patients as better than their expectancies.
Discussion:
By considering that patients had been already pre-treated by at least one chemotherapeutic line and the less responsiveness to chemotherapy of tumour histotypes included in the study, such as the glioblastoma, these preliminary results are clearly surprising with respect to the expected ones in terms of both tumour regressions and duration of response. The results achieved in this study are more promising with respect not only to those expected by chemotherapy alone, particularly in patients with glioblastoma, but also to the results previously described with chemotherapy plus MLT alone, which was already demonstrated to be effective in increasing the efficacy of cancer chemotherapy and reduce its side-effects [10,11], by suggesting a role also exerted by Ang 1-7 in enhancing efficacy and safety of cancer chemotherapy. Moreover, because of the association between clinical response and increase in LMR values, this study would suggest that MLT and Ang 1-7 may enhance the efficacy of chemotherapy at least in part by counteracting the possible chemotherapy-induced immunosuppression, by piloting the immunobiological response of patients in an antitumor way. In any case, it has been shown that both MLT and Ang 1-7may exert direct antiproliferative, cytotoxic and anti-angiogenic effects, which furtherly contribute to chemotherapy-induced cancer cell destruction [14,15]. Therefore, successive randomized clinical trials will be required to confirm the possible enhancing action of Ang 1-7 with respect to chemotherapy plus a concomitant administration of the only MLT.
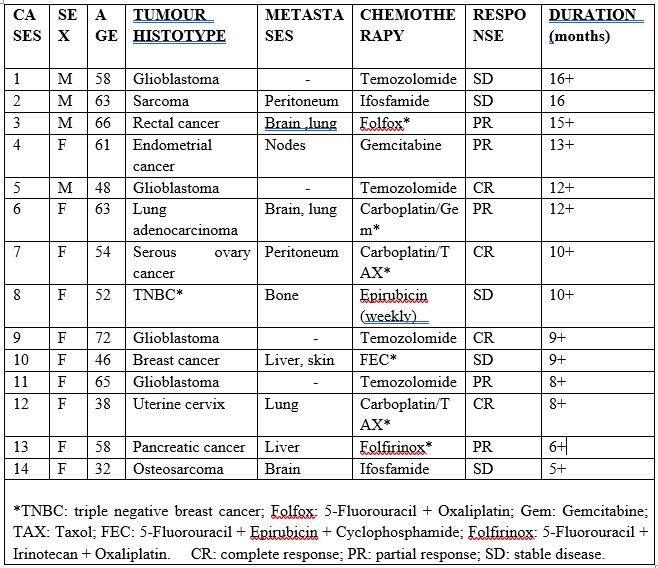
Table 1: Clinical characteristics of advanced cancer patients and their clinical response (WHO criteria) to cancer chemotherapy.