Oncology and Cancer Screening
OPEN ACCESS | Volume 7 - Issue 1 - 2026
ISSN No: 2994-8746 | Journal DOI: 10.61148/2994-8746/JOCS
Gerald C. Hsu
EclaireMD Foundation, US.
*Corresponding Author: Gerald C. Hsu, EclaireMD Foundation, US.
Received: July 29, 2021
Accepted: August 05, 2021
Published: August 10, 2021
Citation: Gerald C. Hsu. (2021) “ Estimated relative cancer risks using metabolism index model, including weighted lifestyle scores and certain metabolic biomarker values from a collected 7-years big data of a type 2 diabetes patient based on GH-Method: math-physical medicine”, J Oncology and Cancer Screening, 3(3); DOI: http;//doi.org/07.2021/1.1037.
Copyright: © 2021 Gerald C. Hsu. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The author recently studied a consensus report published by the American Diabetes Association and the American Cancer Society in 2010 regarding relationships between cancers and diabetes. Based on the learned knowledge from this article and 2+ million collected data of his overall metabolism situation, including both medical conditions and lifestyle details, he decided to conduct a research regarding the estimation of his relative risk levels of having cancers over the past 7 years period from 7/2014 to 7/2021.
This study contains three slightly different weight contribution models of ten cancers related risk factors which are described in the following:
In summary, the three cancer risk waveforms are quite similar to each other in terms of curve shape similarity: 98% correlation of Weighted case vs. Glucose case, and 84% correlation of Weighted cases vs. Equal case. However, the Weighted case shape has shown a lesser degree of shape similarity with the general Metabolism Index (MI) curve shape with a lower correlation of 78%.
Further findings from examining his weighted cancer risk curve in detail, the author was able to identify higher risk regions and lower risk regions.
There are two higher cancer risk periods. The first period includes 2014-2016 due to the earlier years of incorrect handling and insufficient efforts on his diabetes conditions control and overall health improvements. The second period covers 2018-2019 due to his heavy traveling to attend 65+ medical conferences. The two lower cancer risk periods are 2017 due to accumulated health knowledge from his medical research work, and the period of 2020-2021 due to his COVID-19 quarantine lifestyle.
In conclusion, the general trend and relationships between metabolism (not diabetes alone) and cancers are evident:
If this study of past years can shed some light regarding the estimation of the general probability in having cancers during his future years, then it would be able to provide some value to other people for their cancer prevention through metabolism, at least to some degree.
Introduction :
The author recently studied a consensus report published by the American Diabetes Association and the American Cancer Society in 2010 regarding relationships between cancers and diabetes. Based on the learned knowledge from this article and 2+ million collected data of his overall metabolism situation, including both medical conditions and lifestyle details, he decided to conduct a research regarding the estimation of his relative risk levels of having cancers over the past 7 years period from 7/2014 to 7/2021.
This study contains three slightly different weight contribution models of ten cancers related risk factors which are described in the following:
Methods:
Metabolism Index Model:
In 2014, the author applied mathematical topology concept, engineering finite-element method technique, and nonlinear algebra operations to develop a complex mathematical model of metabolism index (MI).
This MI model contains ten specific categories, including four output categories of medical conditions (body weight, glucose, blood pressure, and lipids), and six input categories of lifestyle details (food quantity and quality, drinking water intake, physical exercise, sleep, stress, and daily life routines). These 10 categories are comprised of approximately 500 detailed elements. He has also defined two new resulting parameters: the metabolism index or MI, as the combined score of the above 10 metabolism categories and 500 elements using his developed algorithm, along with the general health status unit (GHSU), as the 90-days moving average value of MI.
A physical analogy of this mathematical metabolism model is similar to “using multiple nails that are encircled by many rubber bands”. For example, at first, we hammer 10 nails into a piece of flat wood with an initial shape of a circle, then take 3,628,800 (=10!) rubber bands to encircle the nails, including all 10 nails. These ~3.6 million rubber bands (i.e. big number of relationships) indicate the possible relationships existing among these 10 nails (i.e. 10 original metabolism data). Some rubber bands encircle 2 nails or 3 nails and so on, until the last rubber band encircles all of these 10 nails together (no rubber band to encircle a single nail is allowed). Now, if we move any one of the nails outward (i.e., moving away from the center of the nail circle), then this moving action would create some internal tension inside the encircled rubber band. Moving one nail “outward” means one of these ten metabolism categories is becoming “unhealthy” which would cause some stress to our body. Of course, we can also move some or all of the 10 nails outward at the same time, but with different moving scales. If we can measure the summation of the internal tension created in the affected rubber bands, then this summarized tension force is equivalent to the metabolism value of human health. The higher tension means the higher metabolism value which creates an unhealthy situation. The author uses the above-described scenario of moving nails and their encircled rubber bands to explain his developed mathematical metabolism model of human health.
Due to the complexity of this particular metabolism construction model, the MI curve possess somewhat different waveform shape from the described three cancer risk cases (Weighted, Glucose, and Equal). These three cancer risk curves are constructed via a much simpler numerical combination of the same 10 calculated MI categories but with different assigned corresponding weighted factors.
From 1/2012 to 7/2021, he has collected more than 2 million data of his own biomedical conditions and personal lifestyle details. Due to concerns of data completion and integrity, he has selected a specific long period from 7/25/2014 through 7/25/2021 which has contained a much more reliable and completed data for this particular cancer risk research.
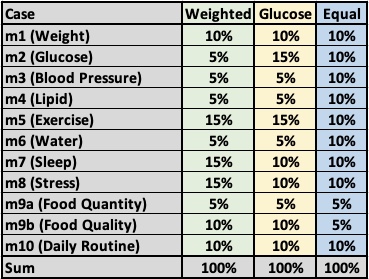
Three cancer risk model:
The three cancer risk cases with different assigned weight-factors are stated in the following data table:
The consensus report of cancer and diabetes:
The following is a rather long excerpt (~2,419 words) from the Reviews/Commentaries/ADA Statements, “Diabetes and Cancer, A consensus report” by Edward Giovannucci, MD, and other authors, published by the American Diabetes Association and the American Cancer Society. The original paper has more than 8,000 words without counting its 123 references. The author considers this paper as a trove of knowledge; therefore, he has kept ~30% of its original words in this excerpt for his future easy access.
“Diabetes and cancer are common diseases with tremendous impact on health worldwide. Epidemiologic evidence suggests that people with diabetes are at significantly higher risk for many forms of cancer. Type 2 diabetes and cancer share many risk factors, but potential biologic links between the two diseases are incompletely understood. Moreover, evidence from observational studies suggests that some medications used to treat hyperglycemia are associated with either increased or reduced risk of cancer. Against this backdrop, the American Diabetes Association and the American Cancer Society convened a consensus development conference in December 2009. Following a series of scientific presentations by experts in the field, the writing group independently developed this consensus report to address the following questions:
1. Is there a meaningful association between diabetes and cancer incidence or prognosis:
Both diabetes and cancer are prevalent diseases whose incidence is increasing globally. Worldwide, the prevalence of cancer has been difficult to establish because many areas do not have cancer registries, but in 2008 there were an estimated 12.4 million new cancer cases diagnosed. The most commonly diagnosed cancers are lung/bronchus, breast, and colorectal, whereas the most common causes of cancer deaths are lung, stomach, and liver cancer (1). In the U.S., the most commonly diagnosed cancers are prostate, lung/bronchus, and colon/rectum in men and breast, lung/bronchus, and colon/rectum in women. Of the world population between the ages of 20 and 79 years, an estimated 285 million people, or 6.6%, have diabetes (2). In 2007, diabetes prevalence in the U.S. was 10.7% of persons aged 20 years and older (23.6 million individuals), with an estimated 1.6 million new cases per year. Type 2 diabetes is the most common form, accounting for ∼95% of prevalent cases (3). Worldwide, cancer is the 2nd and diabetes is the 12th leading cause of death (4). In the U.S., cancer is the 2nd and diabetes is the 7th leading cause of death; Cancer and diabetes are diagnosed within the same individual more frequently than would be expected by chance, even after adjusting for age. Both diseases are complex with multiple subtypes. Diabetes is typically divided into two major subtypes, type 1 and type 2 diabetes, along with less common types, while cancer is typically classified by its anatomic origin (of which there are over 50, e.g., lymphoma, leukemia, lung, and breast cancer) and within which there may be multiple subtypes (e.g., leukemia). Further, the pathophysiologies underlying both cancer and diabetes are (with rare exceptions) incompletely understood.
For more than 50 years, clinicians have reported the occurrence of patients with concurrent diabetes and cancer. However, as early as 1959, Joslin et al. (5) stated, “Studies of the association of diabetes and cancer have been conducted over a period of years, but evidence of a positive association remains inconclusive.” Subsequently, an association between the two diseases was identified in the 1960s in population-based studies. More recently, the results of several studies have been combined for meta-analytic study (6), indicating that some cancers develop more commonly in patients with diabetes (predominantly type 2), while prostate cancer occurs less often in men with diabetes. The relative risks imparted by diabetes are greatest (about twofold or higher) for cancers of the liver, pancreas, and endometrium, and lesser (about 1.2–1.5 fold) for cancers of the colon and rectum, breast, and bladder. Other cancers (e.g., lung) do not appear to be associated with an increased risk in diabetes, and the evidence for others (e.g., kidney, non-Hodgkin lymphoma) is inconclusive.
Diabetes-related factors including steatosis, nonalcoholic fatty liver disease, and cirrhosis may also enhance susceptibility to liver cancer. With regard to pancreatic cancer, interpretation of the causal nature of the association is complicated by the fact that abnormal glucose metabolism may be a consequence of pancreatic cancer (so-called “reverse causality”). However, a positive association between diabetes and pancreatic cancer risk has been found when restricted to diabetes that precedes the diagnosis of pancreatic cancer by at least 5 years.
Only for prostate cancer is diabetes associated with a lower risk. This association has been observed both before and after the advent of screening with prostate-specific antigen (PSA). Some metabolic factors associated with diabetes, such as reduced testosterone levels, may be involved. While obesity has not been associated, and in some studies is even inversely associated, with prostate cancer incidence, obese men with prostate cancer have higher cancer mortality rates than those of normal weight (7). In addition to metabolic factors such as hyperinsulinemia, obesity may be associated with clinical factors (such as delayed diagnosis, poorer treatment) that may underlie the worsened prostate cancer prognosis.
Results of some, but not all, epidemiological studies suggest that diabetes may significantly increase mortality in patients with cancer (8).
Unanswered questions:
Diabetes has been consistently associated with increased risk of several of the more common cancers, but for many, especially the less common cancers, data are limited or absent (6) and more research is needed. Uncertainty is even greater for the issue of diabetes and cancer prognosis or cancer-specific mortality. It remains unclear whether the association between diabetes and cancer is direct (e.g., due to hyperglycemia), whether diabetes is a marker of underlying biologic factors that alter cancer risk (e.g., insulin resistance and hyperinsulinemia), or whether the cancer-diabetes association is indirect and due to common risk factors such as obesity.
In view of the variable associations between diabetes and cancer risk at specific sites, the authors discourage studies exploring links between diabetes and risk of all cancers combined. For example, since lung cancer does not appear to be meaningfully linked with diabetes, including this common cancer in studies will dilute observed associations, should they exist.
2. What risk factors are common to both cancer and diabetes:
Potential risk factors (modifiable and nonmodifiable) common to both cancer and diabetes include aging, sex, obesity, physical activity, diet, alcohol, and smoking:
Nonmodifiable risk factors:
Age.
Although the incidence of some cancer’s peaks in childhood or in young adults, the incidence of most cancers increases with age. In economically developed countries, 78% of all newly diagnosed cancer occurs among individuals aged 55 years and older (11). Diabetes also becomes increasingly common with age: Prevalence is 2.6% in U.S. adults 20–39 years of age, 10.8% in those 40–59 years of age, and increases to 23.8% in those 60 years of age or older (3). In parallel with the obesity epidemic, type 2 diabetes is becoming more frequent among adolescents and young adults (12,13), potentially adding years of additional risk from diabetes to the population.
Sex.
While certain cancers are sex-specific (e.g., cervix, uterine, testicular, prostate), or nearly so (breast), overall cancer occurs more frequently in men. Men have slightly higher age-adjusted risk of diabetes than women (3).
Race/ethnicity.
In the U.S., African Americans are more likely to develop and die from cancer than other race or ethnic groups. Following African Americans are non-Hispanic whites, with Hispanics, Native Americans, and Asian Americans/Pacific Islanders having lower cancer incidence and mortality (14). While incompletely understood, genetic, socioeconomic, lifestyle, and other environmental factors are thought to contribute to these disparities.
Modifiable risk factors:
Overweight, obesity, and weight change:
Overweight (BMI ≥25 and <30 kg/m2) or obese (BMI ≥30 kg/m2) individuals have a higher risk for many types of cancer compared with individuals whose BMI is considered within the normal range (18.5 to <25 kg/m2) (16,17). The cancers most consistently associated with overweight and obesity are breast (in postmenopausal women), colon/rectum, endometrium, pancreas, adenocarcinoma of the esophagus, kidney, gallbladder, and liver. Obesity may also increase risk of mortality from some cancers, such as prostate (7). A growing body of evidence suggests that weight gain is associated with an increased risk of some cancers, breast cancer in particular (17). Increases in body weight during adulthood largely reflect increases in adipose tissue rather than lean mass, so total body fat may be a better measure of the risk for cancer than BMI.
Studies over decades have consistently shown a strong association between obesity and both insulin resistance and type 2 diabetes incidence (18), with risk of diabetes and earlier age at onset directly linked to obesity severity (19). For type 2 diabetes (20) as well as certain cancers (e.g., colon) (21), some studies suggest that waist circumference, waist-to-hip ratio, or direct measures of visceral adiposity are associated with risk independently of BMI.
The case for a causal relationship between obesity and disease is strengthened by evidence that weight loss lowers disease risk. In the randomized, prospective, multicenter Diabetes Prevention Program trial, an intensive lifestyle intervention of diet (targeting 5–7% weight loss) and physical activity was associated with a 58% reduction in diabetes incidence in high-risk individuals (22), and weight loss accounted for most of the effect (23). In addition, weight loss may also limit the risk of developing gestational diabetes (24).
The association between weight loss and subsequent cancer risk is less clear. Weight loss may be a sign of undiagnosed cancer.
Diet:
A majority of studies suggest that diets low in red and processed meats and higher in vegetables, fruits, and whole grains are associated with a lower risk of many types of cancer (17,28,29). Diets that are low in red and processed meat but high in monounsaturated fatty acids, fruits, vegetables, whole grain cereals, and dietary fiber may protect against type 2 diabetes, possibly through improving insulin sensitivity (30,31). Low-carbohydrate diets (which often include greater consumption of red meats and fat) have also been associated with weight loss and improvements in insulin sensitivity and glycemic control. However, randomized controlled trial evidence of dietary interventions and diabetes prevention only exists for low-fat, low-calorie, plus/minus high-fiber diets (22,32).
Several studies suggest that diets high in foods with a high glycemic index or load are associated with an increased risk of type 2 diabetes (28,33). However, evidence of their associations with cancer risk is mixed (28,34,35). Regardless, to the extent that energy-dense and sugary foods contribute to overweight and obesity, the American Cancer Society, the World Cancer Research Fund, and the American Institute for Cancer Research recommend limiting consumption of these foods (17,29).
Physical activity:
Evidence from observational epidemiologic studies consistently shows that higher levels of physical activity are associated with a lower risk of colon, postmenopausal breast, and endometrial cancer (17,36,37). Physical activity may also help prevent other cancers, including lung and aggressive prostate cancer, but a clear link has not been established. Some evidence also suggests that physical activity postdiagnosis may improve cancer survival for some cancers, including breast (38) and colorectal (39).
A protective role for increased physical activity in diabetes metabolism and outcomes has been demonstrated. Data from observational and randomized trials suggest that ∼30 min of moderate-intensity exercise, such as walking, at least 5 days per week substantially reduces (25–36%) the risk of developing type 2 diabetes (40).
Tobacco smoking:
It is estimated that worldwide, tobacco smoking accounts for 71% of all trachea, bronchus, and lung cancer deaths (41). Other cancers strongly associated with smoking are larynx, upper digestive, bladder, kidney, pancreas, leukemia, liver, stomach, and uterine cervix.
Alcohol:
Alcoholic beverage consumption, even in moderate amounts, increases the risk of many types of cancer including those of the oral cavity, pharynx, larynx, esophagus, liver, colon/rectum, and female breast (45).
Unanswered questions:
A critical question is whether the associations between diabetes and risk of certain cancers is largely due to shared risk factors (obesity, poor diet, physical inactivity, and aging), or whether diabetes itself, and the specific metabolic derangements typical of diabetes (e.g., hyperglycemia, insulin resistance, hyperinsulinemia), increase the risk for some types of cancer. While it is clear that lower levels of adiposity, healthy diets, and regular physical activity are associated with reduced risk for type 2 diabetes and for several common types of cancer, these factors are generally interrelated, making the contribution of each factor difficult to assess.
3. What are possible biologic links between diabetes and cancer risk:
Carcinogenesis is a complex process. Normal cells must undergo multiple genetic “hits” before the full neoplastic phenotype of growth, invasion, and metastasis occurs. This process of malignant transformation can be divided into multiple steps: initiation (irreversible first step toward cancer), promotion (stimulation of the growth of initiated cells), and progression (development of a more aggressive phenotype of promoted cells).
Hyperglycemia and cancer:
In considering the complexity of interactions between diabetes, diabetes treatments, and cancer, it is important to not overlook glucose as a potentially relevant mediator. The recent resurgence of interest in the Warburg hypothesis and cancer energetics (66) emphasizes the dependence of many cancers on glycolysis for energy, creating a high requirement for glucose (or even “glucose addiction”)
Insulin receptor activation may be a more important variable than hyperglycemia in determining tumor growth.
Major unanswered questions:
As previously outlined, there is a growing body of epidemiologic evidence supporting a link between diabetes and the incidence and/or prognosis of some cancers. It is recognized the association may not be causal; diabetes and cancer may be associated simply because they share common predisposing risk factors such as obesity.
Individuals with type 1 diabetes represent ∼5% of the diabetes population worldwide. The autoimmune destruction of the pancreatic β-cells results in the loss of insulin production and the need for immediate and lifelong insulin therapy. In contrast, type 2 diabetes is much more common and accounts for ∼95% of the diabetes population. Type 2 diabetes is generally associated with overweight and obesity (in an estimated 80% of cases) and commonly advances from a pre-diabetic state characterized by insulin resistance (hyperinsulinemia) to frank diabetes with sustained insulin resistance accompanied by a progressive reduction in insulin secretion.
Insulin and insulin analogs:
Insulin is required for all patients with type 1 diabetes. It is also necessary for many patients with type 2 diabetes to treat hyperglycemia, in part due to the progressive loss of β-cell function over time. Between 40–80% of individuals with type 2 diabetes will ultimately be considered for insulin therapy in an effort to achieve glycemic targets (77).”
The author’s learned key-points from the consensus report:
After reading this consensus report four times, the author attempts to derive some conclusive learning from this paper. The selection of different weight-factors for 10 metabolism categories of these 3 cancer risk calculations are derived based on the following key-points of his learning.
Results:
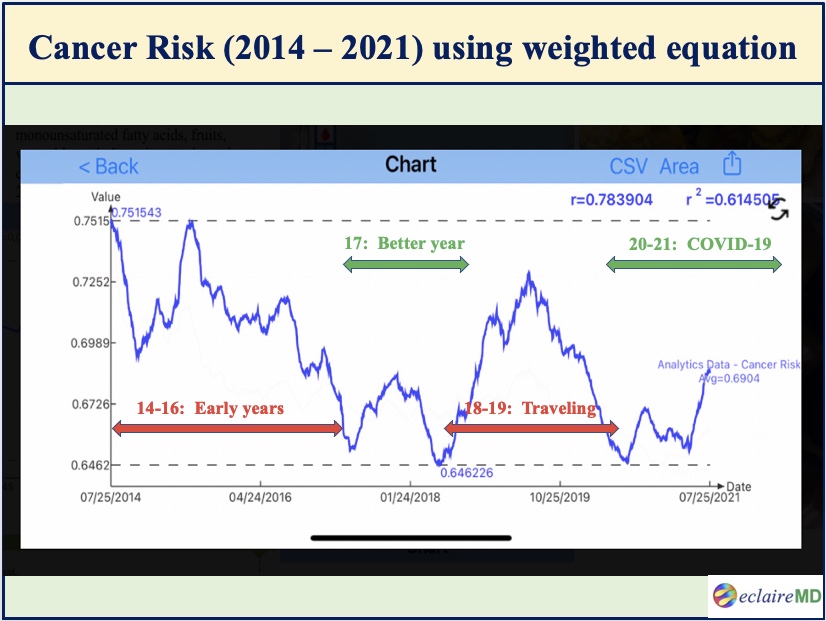
Figure 1 shows the main result of this study which is the cancer risk curve during the period from 7/25/2014 to 7/25/2021. The Y-axis magnitude is not the “absolute” risk of having cancers but rather a “relative” risk scale of developing various cancers.
In Figure 1, the green horizontal timespans have lower cancer risks while the red horizontal timespans have higher cancer risks:
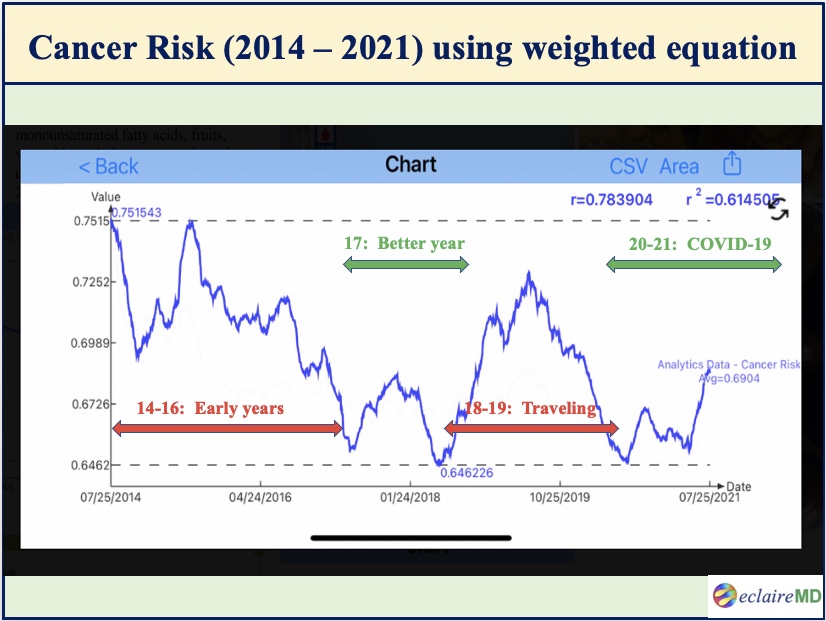
Figure 1: Cancer risk curve using different weighted factors for 10 metabolism categories (7/2014 -7/2021)
Looking into this particular cancer risk waveform, it is obvious that 2014-2016 and 2018-2019 have relatively higher risks, while 2017 and 2020-2021 have relatively lower risks.
After self-studying internal medicine and food nutrition for 4 years during 2010-2013, the author started to conduct his own medical research on metabolism and diabetes since 2014. He further developed several prediction models of weight, glucoses, and HbA1C during the period of 2015-2016. Therefore, his overall knowledge learned regarding health and diseases were still pre-mature during this early stage.
During the two-year period of 2018-2019, he traveled to 50 international cities to attend 65+ medical conferences and made 120+ oral presentations of his written medical papers. This heavy and hectic traveling schedule has disturbed his routine lifestyles and also inflicted damages to his health to some degree.
His best-controlled years are 2017, 2020, and 2021 because they are a direct result of his learned knowledge, disciplined lifestyle, and peaceful life-routines. In addition to the productivity on his medical research work, the COVID-19 epidemic and its associated quarantine life have actually turned out to be beneficial for him to further his health improvement and diabetes disease control.
Figure 2 reveals three comparison results as outlined below, using the correlation coefficients (R):
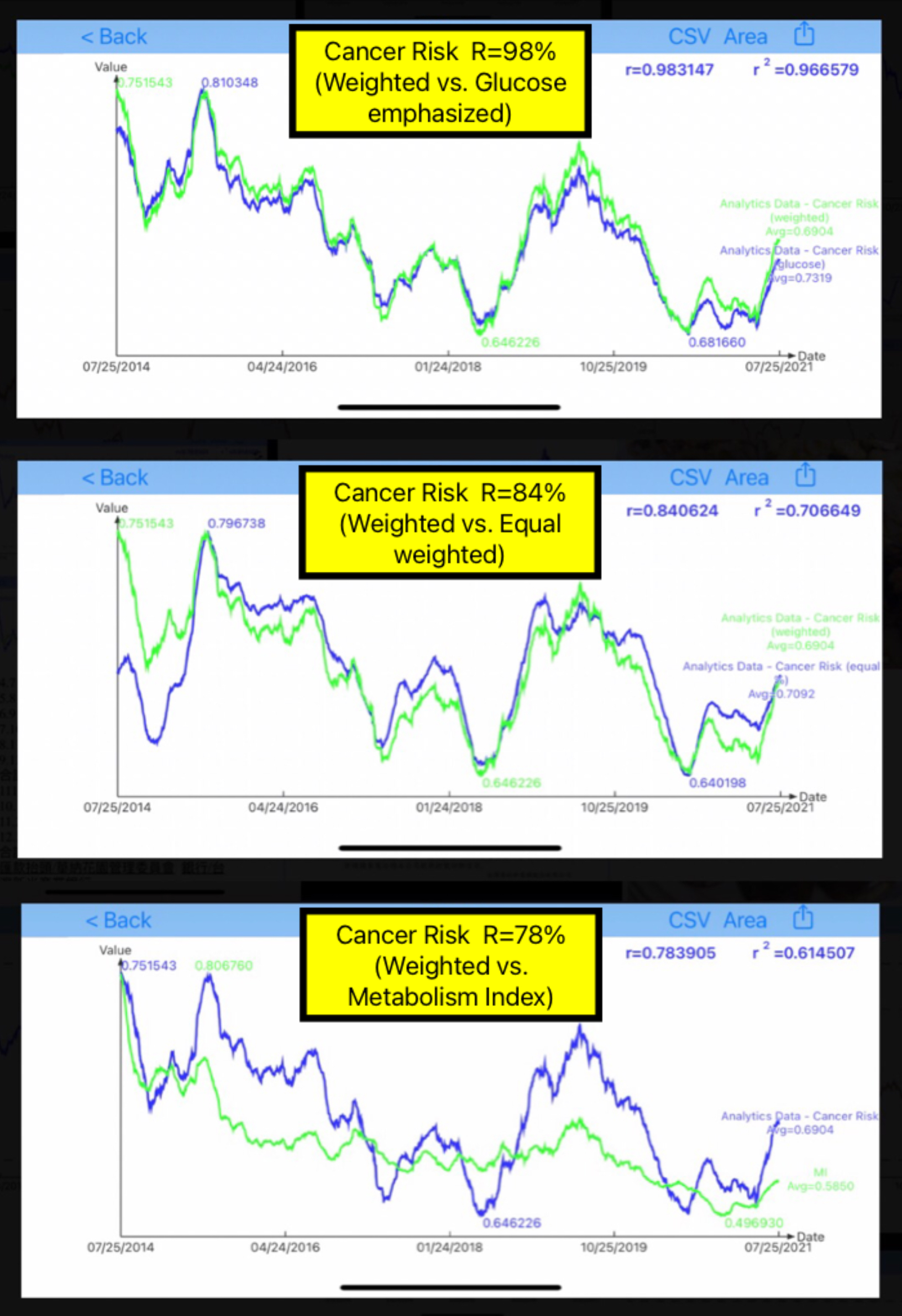
Figure 2: Comparison of Weighted case versus Glucose-emphasized case, Equal-weights case, and MI curve
Weighted vs. Glucose: R=98%
Weighted vs. Equal: R=84%
Weighted vs. MI: R=78%
Generally speaking, the results from the 3 cancer risk cases are quite similar to each other. However, the cancer risk curve and the MI curve have some visible differences due to his Metabolism definition and the build-in complexity of MI model construction using inter-relationships among 10 different metabolism categories in a nonlinear space. Nevertheless, the general trend and relationships between metabolism and cancer are clearly evident.
Conclusion:
In summary, the three cancer risk waveforms are quite similar to each other in terms of curve shape similarity: 98% correlation of Weighted case vs. Glucose case, and 84% correlation of Weighted cases vs. Equal case. However, the Weighted case shape has shown a lesser degree of shape similarity with the general Metabolism Index (MI) curve shape with a lower correlation of 78%.
Further findings from examining his weighted cancer risk curve in detail, the author was able to identify higher risk regions and lower risk regions.
There are two higher cancer risk periods. The first period includes 2014-2016 due to the earlier years of incorrect handling and insufficient efforts on his diabetes conditions control and overall health improvements. The second period covers 2018-2019 due to his heavy traveling to attend 65+ medical conferences. The two lower cancer risk periods are 2017 due to accumulated health knowledge from his medical research work, and the period of 2020-2021 due to his COVID-19 quarantine lifestyle.
In conclusion, the general trend and relationships between metabolism (not diabetes alone) and cancers are evident:
If this study of past years can shed some light regarding the estimation of the general probability in having cancers during his future years, then it would be able to provide some value to other people for their cancer prevention through metabolism, at least to some degree.