Oncology and Cancer Screening
OPEN ACCESS | Volume 7 - Issue 1 - 2026
ISSN No: 2994-8746 | Journal DOI: 10.61148/2994-8746/JOCS
Alkalás Pérez B 1, Eduardo León Lezcano 2, Ana L.Osejo 3 , Bosco E. Lazo 3 , José M. Hurtado 4 , Donoso Peñalba-Rivera 5 , Orestes López Piloto 6
1National Neurosurgery Service, Hospital Escuela Antonio Lenin Fonseca, Managua, Nicaragua. Neurosurgeon, Coordinator of the Residency Program.
2Pathology Service, Antonio Lenin Fonseca.
3Resident of the National Neurosurgery Service, Neuro Oncology Interdisciplinary Group Committee.
4Neurosurgeon, Manuel de Jesús Rivera Pediatric Hospital, Managua, Nicaragua.
5Professor Department of Public Health, Universidad Nacional Autónoma de Nicaragua. León.
6Consultant Professor of Neurosurgery. Neurology and Neurosurgery Institute. Havana. Cuba
*Corresponding Author: Orestes López Piloto, Consultant Professor of Neurosurgery. Neurology and Neurosurgery Institute, Havana Cuba.
Received: March 11, 2021
Accepted: March 16, 2021
Published: March 22, 2021
Citation: Orestes L Piloto, Giant Aneurysmal Bone Cyst on Temporal Bone: Case report and review. J Oncology and Cancer Screening, 2(1); DOI: http;//doi.org/03.2021/1.1006.
Copyright: © 2021 Orestes López Piloto. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Objective and Importance: We present a rare case of a giant Aneurysmal Bone Cyst (ABC) of the temporal bone that caused brain herniation and was treated with surgery, highlighting the role of near total or total resection for the control of disease. Currently without progression at the residual tumor sites of the areas of vascular and neural involvement on the skull base.
Clinical Presentation: Male, 23 years old who presented with motor impairment and signs of intracranial hypertension due to brain herniation associated with a giant bulging mass and pain in the right temporal region, which was ABC.
Intervention: Wide craniectomy with near total resection, limited by extension and osteolysis in petrous bone.
Conclusion: ABC that affects the skull is infrequent and near or total resection allows control of disease, without progression, except when bones of the skull base with vascular and neural structures are involved. Adjuvant therapies may be necessary.
Background:
Aneurysmal Bone Cyst (ABC) is an osteolytic, expansive, hemorrhagic, hyperemic and pseudo tumoral bone lesion [9]. It is a rare entity that represents only 1% - 2% of all bone tumors2. Different pathogenic theories have been proposed. Among the most accepted is the idea that the primary injury (trauma) gives rise to the formation of a periosteal or intraosseous fistulous arteriovenous connection; while the secondary lesion is formed by arteriovenous fistula in a pre-existing benign primary bone pathology [1].
The skull is a rare location and represents only 3% to 6% of all ABC [10]. Twenty-one cases of ABC of the temporal bone have been described in the literature [17]. ABC can affect any bone segment, with the long bones (67% of cases) being the most frequently involved [10].
Surgical resection of the ABC shows a good local control rate with an excellent general prognosis, although this treatment can be affected by complications such as bleeding, pain, and alterations in bone growth. The risk of local disease progression after subtotal resection is as high as 25%, generally within the first 2 years after treatment [10].
Clinical presentation:
Male, 23 years old with a denied chronic pathological history but who reports mild Brain traumatic injuries several times before his symptoms began. He came to the Neurosurgery department of our hospital after noticing an asymmetric growth of his skull with a bulging shape of the right temporal bone for about 4 years. It causes headache of moderate intensity, oppressive and aggravated by palpation at the affected site. He refers that 4 months ago, he noticed weakness in the left side that did not improve. He also associates slight ipsilateral ocular proptosis.
Due to presenting left hemiparesis, he decided to go for evaluation. On physical examination, normal cognitive functions; 14 points on the Glasgow coma scale; without impairment of eye movements or vision on the right side. Bilateral papilledema. Left supra-nuclear facial palsy was observed, without hearing loss; with 3/5 left hemiparesis and hyperreflexia.
Hematological and biochemical profiles in normal parameters.
The cranial computed tomography (CT) scan revealed the right cerebral hemisphere compressed by a mass that appeared to emerge from the right mastoid and that expands the diploe towards the midline and a superior and anterior direction, with partially calcified thin borders. Inside, it has liquid density with multiple trabeculae that do not enhanced with contrast. The tumor measures approximately 91 x 63 x 75 mm. Part of the temporal bone in contact with this tumor is thinned and eroded, without affecting the overlying soft tissues. There is subalpine herniation, with peri mesencephalic and prepontine obliterated cisterns. No vascular lesions (Fig. 1).
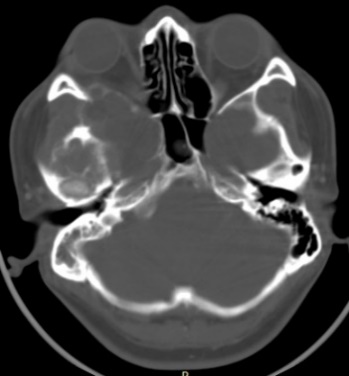
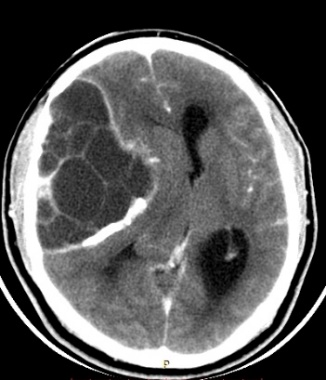
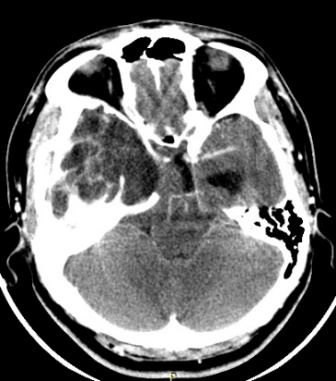
Figure 1: Pre-operative cranial tomography (CT) in axial section with brain and bone windows showing subalpine herniation from right to the left caused by a mass on the floor of the middle fossa, that expands the diploe, with partially calcified edges. Inside, it has a liquid density with multiple trabeculae that enhanced with the contrast. Portions of the temporal bone are thinned and eroded, without affecting the overlying soft tissues.
In the cranial magnetic resonance images (MRI) the lesions are extra-axial, weighted in T1 showed to be isointense to hyperintense with relation to Gadolinium and the T2-weighted images showed a destructive lesion of mixed signal intensity, hyperintense inside septate cyst mode, extending into the middle cranial fossa, petrous bone and right mastoid cells.
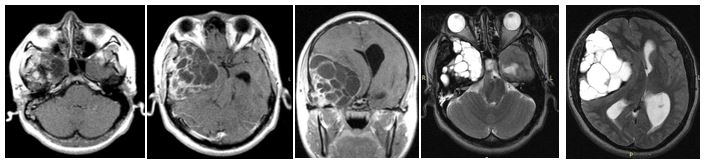
Figure 2: Cranial Magnetic resonance images (MRI) in axial and coronal sections, in contrasted T1, showing isointense extra axial lesion that enhanced with the contrast in his trabeculae and solid areas. The axial T2 images showed a destructive lesion of mixed signal intensity that extended towards the middle cranial fossa, petrous pyramid and mastoid cells; hyperintense inside as septate cysts.
Studies to rule out primary neoplastic lesions were normal.
The patient underwent resection of the tumor by craniectomy, finding a heterogeneous lesion whose cystic content was xanthochromia liquid; solid localized epidural areas arising from bone tissue; very vascularized and bleeding. The squamous portion of the temporal bone was thin, brittle, and eroded.
On histopathological examination, the tumor was composed of non-meningothelial mesenchymal tissue, blood-filled spaces of different sizes separated by wide fibrous septa. The septa were composed of freely arranged oval or spindle cells with interspersed multinucleated giant cells. Cellular infiltrate, focal areas of osteoid material production; hemorrhage area and hemosiderin-laden macrophage aggregates. No evidence of malignancy was demonstrated. The histopathology of ABC was confirmed (Fig. 3).
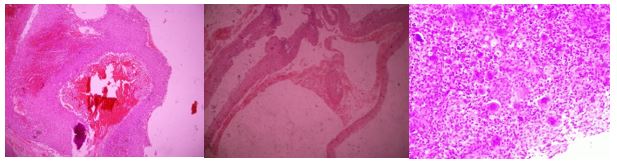
Figure 3: Mesenchymal tissue, blood-filled spaces separated by wide fibrous septa. Cellular infiltrate, focal areas of osteoid material production; hemorrhage areas and hemosiderin-laden macrophage aggregates.
In the postoperative period, there is resolution of the left hemiparesis with improvement of the ipsilateral central facial palsy. Laboratory tests showed leukocytosis and anemia as postoperative changes that were treated accordingly.
The CT scan in the immediate postoperative period shows a frontal temporal craniectomy area with improvement of the subalpine herniation. Post-surgical changes are observed in relation to pneumocephalus in the tumor bed. Areas of calcification attached to the dura. The persistence of an osteolytic lesion is evidenced in the floor of the middle fossa that partially involves the petrous portion of the temporal bone (Fig. 4).
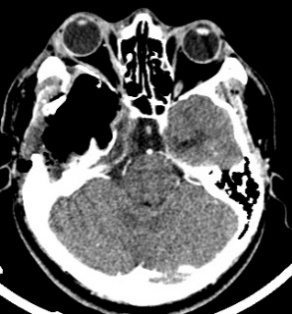
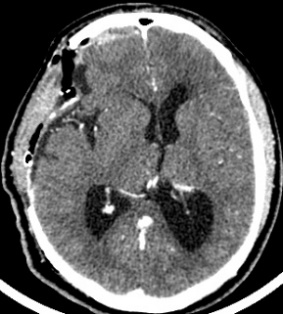
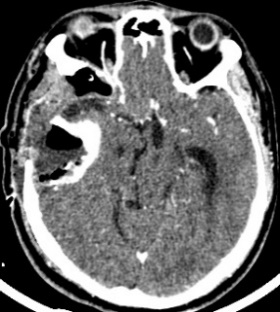
Figure 4: Cranial CT in axial sections showing decreased brain herniation; area of craniectomy and near total resection of the tumor but persistence of the same in the petrous portion of the temporal bone.
Ten months after surgery, the patient was asymptomatic, considering the presence of a residual lesion at the petrous bone, adjacent to the carotid artery and semicircular canals. Invariable with respect to immediate post-surgery, for which vigilance and expectant management are decided. No progression was observed (Fig. 5).
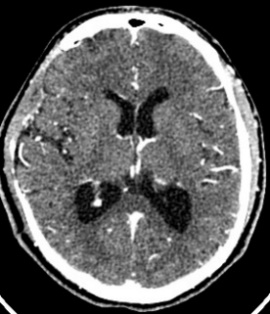
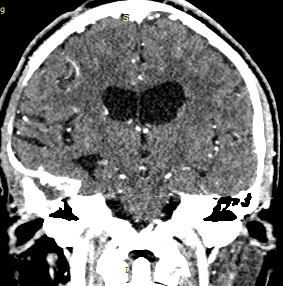
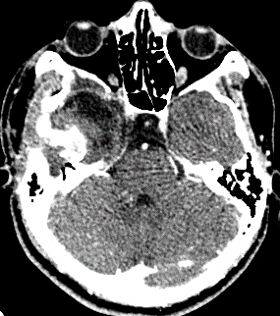
Figure 5: cranial CT scan with axial and coronal sections in contrasted phase at 10 months postoperative, showing area of right frontal temporal craniectomy and residual lesion at the level of petrous bone without regrowth
Discussion:
The term Aneurysmal Bone Cyst (ABC) was first used in 1942 by Jaffe and Lichtenstein [12] to describe two examples of blood-filled cysts in which the tissue from the cyst wall contained conspicuous spaces, areas of hemosiderin deposition, giant cells and occasional bone trabeculae [21]. This term coined by Jaffe and Lichtenstein, however, is a misnomer as this lesion is neither an aneurysm nor a cyst [8].
ABC is a pseudo tumoral hyperemic hemorrhagic expansive osteolytic bone lesion9. It is a rare entity, representing only 1% - 2% of all bone tumors2. Although ABC may occur at any age, it prevalently appears in early childhood and adolescence with a slightly female preponderance [22].
Macroscopically, the lesion consists on multiple cavities, lacking an endothelial lining, separated by thin fibrous septa and usually filled with blood [23].
ABC can occur virtually in any bone but most commonly affects femur and tibia.
The cranium is a rare location and accounts for only 3 to 6% of all ABC [10]. Symptoms and signs corroborate with the site of the disease. The radiological hallmark of ABC is the ballooned-out appearance and eccentricity. Management usually consists of wide local excision. Twenty-one cases of ABC of temporal bone have been reported in the literature [17].
ABCs may affect any bone segment, with most affected sites being the long bones (67% of cases), spine (15%), and pelvis (9%). Within the spine, most commonly ABCs affect the posterior elements of lumbar segments, followed by cervical and then thoracic vertebrae13. The presenting symptoms, when the tumor is in the cranium, are usually that of a painful swelling of several months, focal neurological deficits, proptosis, signs of increased intracranial tension, headache and conducting hearing loss, depending upon the location of the tumor17. A bruit may be transmitted from a large ABC. The progressive enlargement of cyst in the vertebral column can cause cord and root compression leading to neurological deficit [11].
Pathogenesis remains controversial, though different theories have been proposed. Among the most accepted ones is the idea that the primary lesion (sometimes injury) start a periosteal or intraosseous fistulous arteriovenous connection. Highflow through the lesion erodes the osseous trabeculae into a cystic cavity. The rapid bony remodeling, in response to the hemodynamic forces, results in the lesion having a ballooned, thin-shelled, and multiseptated soap-bubble form [4].
These findings led to assumption that ABC may be due to “blow out” of hemorrhage in cases of elevated pressure or “spurt of blood” in cases of under pressure into preexisting benign lesions of the bone, namely chondroblastoma, fibrous dysplasia, fibrosarcoma, and unicameral bone cyst10. Thus, ABC has been considered as secondary reactive lesion caused by arteriovenous fistula in a preexisting primary benign pathology of the bone [1].
ABCs may be either primary namely when they arise de novo or secondary when they coexist with other lesions such as ossifying fibroma, chondroblastoma, solitary bone cyst, giant cell tumor of the bone, osteosarcoma, osteoblastoma, giant cell reparative granuloma, fibrous dysplasia, and fibromyxoma12. May differ from a molecular standpoint, with the primary ABC showing USP6 or CDH11 rearrangements in approximately two thirds of cases [23].
Recent cytogenetic data reveals that primary ABC or “classical” it is considered an independent neoplasia because it is correlated with a specific genetic translocation (16;17) (q22;p13) involving the gain of function of TRE17/USP6 (Ubiquitin-Specific Protease 6 gene) which results in the formation of CHD11-USP6 fusion transcripts. It is normal to find CHD11 in strong calcified bone, however, through oncogenic mechanisms, ABC patients express deregulate USP6 genes14.
Won Jong, Bank; in his classic histopathologic study of aneurysmal bone cyst with 215 cases, including 101 primaries and 114 secondary cases; revealed that Blue Reticulated Chondroid-like material (BRC) is characteristic of ABC. BRC appears to be a unique histopathologic feature of ABC, making it valuable to differentiate benign ABC from telangiectatic osteosarcoma in hematoxylin – eosin stain24.
Callotian, Sean reported an extremely rare pathology of concomitant polyostotic Fibrous Dysplasia (FD) with simultaneous ABC of the skull/skull base, homers and rib. In literature, there exists many cases of polyostotic FD, but no cases demonstrate concomitant occurrence of FD with ABC formation in three different locations throughout the body. His report represented a novel case with multiple FD lesions with secondary ABC, highlighting the importance for a complete preoperative workup to determine the presence and extent of all lesions [19].
Also, the development of secondary ABC in Langerhans Cell Histiocytosis (LCH) is rare, with only two reported occurrences in the skull in the literature. The presence of lesions with ABC like radiographic findings in bones that are also typical sites for LCH should be submitted to immunohistochemical analysis (CD1a, S100 and Langerin stains) to test for LCH involvement [15].
Plain x-rays, classically, presents as ballooned or aneurysmal cystic expansion with surrounding rim of sclerosis and periosteal new bone formation18. CT scan shows multiloculated lytic lesions and fluid levels and defines the extent of the bony destruction, the integrity of the cortical bone. Gadolinium administration can help to differentiate the thin, smooth ABC septations from other masses that are neoplastic in nature and instead have nodularity [3].
Treatment of ABCs remains debated. Options include surgery, aiming to intralesional curettage or complete excision; endovascular procedures with arterial embolization; percutaneous intralesional drug injection; pharmaceutic intervention; and radiation. The surgical excision of the ABC shows good local control rate with excellent overall prognosis, though this treatment may be burdened by complications, such as bleeding, pain, growth disturbances, and risk of vertebral instability or deformity needing spinal stabilization. If the risk of surgical excision is too high because of difficulty accessing sites or structurally significant regions, curettage and bone grafting may be preferred. The risk of local disease progression after subtotal resection is as high as 25% in largest clinical series, usually within the first 2 years after treatment [7].
On these grounds, chemical or physical adjuvants, such as phenol, bone cement, cryotherapy with liquid nitrogen, or the mechanical effect of a high-speed drill, have been used to reduce the risk of local recurrence after excision. However, significant differences among the results of these options have not been reported so far.
Radiation therapy has been usually limited as adjuvant therapy to patients with inoperable lesions, high surgical risk, recurrent disease or after incomplete excision7, due to the risk of complications as secondary malignancies16. Radiation therapy was not preferred in the past due to the potential for malignant transformation of these lesions. Most of them were usually treated with suboptimal doses of radiation using orthovoltage equipments, which are now obsolete. However, in the present era with the megavoltage therapy, adequate and homogenous radiation doses can be precisely delivered to the site of the lesion. The dose prescribed ranged from 20-60 Gy. No local recurrence was seen in the median follow up of 17 years (range, 20 months - 20 yr)17.
Kumar et al, view of the response evident in his patient, radiotherapy seems to be effective for recurrent cases of ABC at the temporal bone and a dose of around 30 to 36 Gy could be effectively delivered with satisfactory results17.
Embolization has been used as neoadjuvant or adjuvant treatment of open surgery, in order to minimize the bleeding during excision and further reduce the risk for local recurrence [6].
Current evidence favors sclerotherapy as the chosen initial treatment for ABC in the absence of compression of neural structures4. Several agents have been proposed to inject in order to promote healing of the ABC and reduce the recurrence rate. Polidocanol requires multiple procedures and is not approved in the United States. Doxycycline has been used in combination with a foaming agent but may require multiple injections20. Ethanol has a simple manipulation formula and has been recently used alone or in combination with Surgiflo (Ethicon, Somerville, New Jersey, USA) [5].
Conclusion:
The Aneurysmal Bone Cyst is common in long bones but few injuries affecting the skull were reported, as it occurs in this case, where it reaches giants dimensions capable of causing brain herniation with focal symptoms.
Near total or total resection allows resolution of the clinical, control of the disease, no progression.When the bones of the skull base with their vascular and neural structures are involved, total resection is limited by the morbidity and mortality involved. Adjunctive therapies may be necessary when progression was observed.