Mohini Agrawal
Department of Ophthalmology,Command Hospital,Pune, India.
*Corresponding Author: Mohini Agrawal, Department of Ophthalmology,Command Hospital,Pune, India.
Received Date: September 15, 2023
Accepted Date: October 22, 2023
Published Date: November 28, 2023
Citation: Mohini Agrawal. (2023) “A Rare Presentation of Orbital Inflammation following Zoledronate Infusion”, Ophthalmology and Vision Care, 4(1);
DOI: 10.61148/2836-2853/JOVC/043.
Copyright: © 2023 Mohini Agrawal. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Zoledronate, a third-generation bisphosphonate, is commonly used as an annual intravenous infusion, primarily in the treatment of osteoporosis. Ocular adverse effects have been documented and orbital inflammation is one of the most severe. It may clinically manifest by eyelid edema, conjunctival congestion, chemosis, restricted ocular motility, pain, diplopia, and reduced vision. It usually has asymmetric involvement and rarely associated with anterior uveitis, scleritis, episcleritis or conjunctivitis. Prompt treatment is likely to have favorable outcomes. Though, the treatment of choice remains systemic corticosteroids, we highlight a case who developed orbital inflammation after Zoledronate-infusion and improved after topical corticosteroids administration.
orbital inflammation; zolendronate; corticosteroids; bisphosphonates
Introduction:
Bisphosphonates are commonly used in the treatment of diseases like osteoporosis, Paget’s disease, osteoclastic bone metastases, hypercalcemia and multiple myeloma. [1-2] The most commonly seen ocular side-effects pertaining to bisphosphonates are conjunctivitis, anterior uveitis, episcleritis and scleritis.[3] There are also other serious inflammatory side-effects, namely that of orbital inflammatory disease arising from the use of bisphosphonates. Systemic corticosteroids are usually the treatment of choice. Zoledronate is a third-generation bisphosphonate and one of the most commonly used bisphosphonates as it permits an annual dosing regimen by a 15-minute intravenous infusion.
Till date, fewer case reports have been documented on bisphosphonates-associated orbital inflammation; here we intend to add an additional case of orbital inflammation associated with zoledronate infusion, that was successfully treated with the administration of topical corticosteroids.
Case Report:
A 65-years-old male underwent total left hip replacement for fracture neck femur in May 2016 followed by 5mg intravenous infusion of zoledronic acid on June 2016 for osteoporosis. The patient complaint of acute-onset ocular redness, pain and diminution of vision in left eye (LE) 72-hours following the infusion. His medical history was otherwise unremarkable.
On examination, his best-corrected visual acuity (BCVA) was 6/12 in left eye. The slit lamp exam was remarkable for marked swelling of eyelids, conjunctival congestion and chemosis in left eye (Figure 1a); rest of the anterior segment and posterior segment was normal. Right eye (RE) had no abnormality. Intraocular pressures were also normal.
Based on clinical diagnosis, the patient was treated presumptively as orbital cellulitis LE with intravenous antibiotics and supportive treatment. On the second day, the patient presented with worsening of symptoms and developed proptosis of LE (Hertel exophthalmometry RE 20mm and LE 23mm). On Day-3 the patient complaint of redness and swelling in the RE also, but of lesser severity. In view of bilaterality and a possible diagnosis of cavernous sinus thrombosis, the patient was advised urgent MRI scan which showed left periorbital soft-tissue swelling and post-septal fat stranding with proptosis in LE. There was no orbital collection or signs of sinusitis.
Despite high-dose intravenous antibiotics for 5 days, there was no improvement. So, based on the clinical presentation, MRI findings and timing of symptoms, a diagnosis of orbital inflammatory syndrome following zoledronic acid infusion was made. The patient was started on topical corticosteroids. On Day-6, the chemosis reduced and proptosis started resolving. The patient responded to the treatment, chemosis and congestion completely resolved and visual acuity returned to 6/6 in both the eyes by day-10 (Figure 1b).
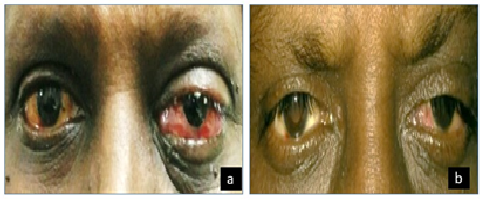
Figure 1: (a) External appearance of the left eye with proptosis, ptosis, and conjunctival chemosis 72-hours following bisphosphonate infusion; (b) Complete resolution of ocular signs at day10 after treatment with topical steroids.
Discussion:
Most of the patients tolerate bisphosphonates and seldom develop serious side effects. Ocular complications like anterior uveitis, scleritis, episcleritis, conjunctivitis or orbital inflammation are less frequent and the exact mechanism is also not well understood.[4] However, number of experts consider ocular inflammation as part of an acute-phase response by activation of gamma delta T-cells and release of cytokines like interleukin-1 and 6.[5]
Many of the reported cases of bisphosphonate-induced acute orbital inflammation in the literature occurred following zoledronic acid infusion. [6-10], although the other bisphosphonates can also lead to ocular adverse effects.[3] The intravenous route and yearly dosing are attractive alternatives to patients since oral bisphosphonates must be taken with a full glass of water while fasting and the patient must remain upright for at least 30-min after ingestion. Zoledronate has a higher potency and has actually been effective in once-yearly dosing for managing osteoporosis.[10]
In the previous published literature, ocular inflammation occurred after the first infusion of zoledronic acid and the symptoms develop within 3 days of medication intravenous administration. [6-8] Similarly, our case highlights the rare complication of zoledronic acid infusion. The symptoms occurred within 72-hours of the infusion and the patient presented with frequently seen symptoms like ocular pain, orbital swelling, redness, and diminution of vision. Our patient responded very well to topical corticosteroids.
Due to the grave nature of many of the indications for bisphosphonate, the likelihood of continuing treatments inspite of developing orbital inflammation is likely to be clinically significant for some patients. Each case should be well-thought-out individually and should involve a vigilant discussion with the patient regarding the risks and benefits of continued treatment and other options that may be available.
This case adds to the scarce literature on orbital inflammation associated with Zoledronate infusion. Nevertheless, this case highlights that though the treatment of choice is systemic corticosteroid, topical corticosteroid may also help in resolution of signs and symptoms induced after bisphosphonate infusion. It is important to identify these adverse effects for proper management as the presentation may mimic more common diseases like orbital cellulitis and cavernous sinus thrombosis and the management is entirely different.
Conclusion:
Orbital inflammation is a rare, but serious side-effect seen after treatment with bisphosphonate infusion. The upsurge may be due to the more extensive use of bisphosphonates or simply due to improved awareness among physicians. Thus, it is important for ophthalmologists and physicians to understand the association between orbital inflammatory disease and bisphosphonates use. The use of topical steroids may also be considered as an alternative treatment for those patients where systemic corticosteroids are contraindicated.
Acknowledgement: Nil
Declaration of interest statement: The authors report there are no competing interests to declare.
Source of funding: Nil
Conflict of interest: Nil