Ophthalmology and Vision Care
OPEN ACCESS | Volume 4 - Issue 1 - 2025
ISSN No: 2836-2853 | Journal DOI: 10.61148/2836-2853/OVC
Fahmy RM1,2* and Nada Al Bahloul1
1Department of optometry and vision sciences, College of applied medical sciences, King Saud University, Saudi Arabia.
2Department of ophthalmology, Faculty of medicine, Cairo University, Egypt.
*Corresponding Author: Fahmy RM, Department of optometry and vision sciences, College of applied medical sciences, King Saud University, Saudi Arabia.
Received Date: June 06, 2022
Accepted Date: July 15, 2022
Published Date: September 26, 2022
Citation: Fahmy RM and Nada Al Bahlou. (2022) “Influence of active and passive smoking on the tear film in the Saudi population”, Ophthalmology and Vision Care, 2(2); DOI: http;//doi.org/06.2022/1.10231
Copyright: © 2022 Fahmy RM. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly Cited.
Purpose:
To investigate the effects of different types of smoking on the precorneal tear film stability and quality using questionnaire, clinical tests and non invasive keratography. And to provide community awareness about the dangers of dry eye and its connection to smoking.
Methods:
Prospective cross-sectional study with a total of 33 participants was enrolled in this study. The participants' ages ranged between 17 to 40 years old. They were divided into 3 groups: Control nonsmokers, Passive smokers, and Active smokers. All participants of control and passive smokers groups are females (100.0%), while there are (45.5%) male participants in active smokers group and (54.5%) females. there were no statistically significant differences between groups (non – passive - active) smokers related to spherical equivalent; p value was (0.403). All subjects replied to questionnaire for dryness evaluation then went through full ophthalmological examinations. Also, we examined subjects for dryness using clinical tests (Tear break up time TBUT and Schirmer) and non-invasive keratography.
Results:
Dry Eye Questionnaire analysis: all control group participants were normal and not dry. There are (54.5%) dry, while (45.5%) in passive Group were not. And participants of active smokers group with percentage of (54.5%) were dry, (45.5%) were not dry. As regards clinical parameters (TBUT and Shirmer): we concluded that there were no statistically significant differences between groups related to TBUT & Schirmer tests, where p value was 0.095, 0.993 respectively.
Non invasive keratograph dryness tests (TBUT & tear meniscus height TMH) we concluded that there were statistically significant differences between groups related to TBUT where p value was 0.026, with insignificant reduction of TMH values in active smoker group as P value was 0.283.
Conclusion: These findings suggest that chronic smoking has a negative effect on the ocular surface and affects some tear characteristics. Moreover, the chronic ocular irritative effects of cigarette smoking may lead to defects in ocular surface defense
Introduction:
The tear film is a pre-ocular, thin, complex and moist structure composed of four layers (lipid layer 0.1 µm, aqueous layer 7 µm, mucous layer 3–30 µm and glycocalyx 0.01–0.02 µm from anterior to posterior) that covers the cornea, bulbar and palpebral conjunctiva [1,2,3]. Any abnormalities to its structure will affect ocular surface and may alter corneal clarity [4]. It has optical, mechanical, nutritional, and defensive functions [5].
Tear film total volume is 7–10 µl. Normal basal tear secretion rate is 1–2 µl/min; on the other hand, the reflex tear rate is >100 µl/min [6]. Normal tear volume replacement occurs every 5–7 min (3). Tear film thickness (TFT) is around 5.35 µm however the central TFT value was 5.122 ±0.034 µm [7].
International Dry Eye Workshops (DEWS 2007) provided the following definition: “Dry eye is a multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability with potential damage to the ocular surface. It is accompanied by increased osmolality of the tear film and inflammation of the ocular surface” [8].
Dry eye disease is classified to two major classes’ aqueous deficient dry eye and evaporative dry eye. Both lead to tear hyperosmolarity [9].
Active smoking has been linked to a variety of systemic and ocular disorders. It has been associated with cataract [10], and age-related macular degeneration [11]. Moreover, Carbon monoxide, methanol, aldehydes, nitrosamines, hydrocarbons, and heavy metals are all hazardous components found in tobacco smoke [12]. Environmental tobacco smoking (ETS), commonly known as passive smoking, can harm the eyes which is particularly vulnerable to air contaminants due to its exposure to the environment. Dry eyes, burning sensation, and itching are another prominent impact of cigarette smoke exposure [13]. Recently, there is an increase in the use of electronic cigarettes (ECs), composed of a battery, a flow sensor, an atomizer, and a coil with the active liquid in it. EC vapors also contain a significant amount of free radicals that are irritant to the eye [14].
Dry eyes can be diagnosed non-invasively using non-invasive tear film break up time (NITBUT) and tear meniscus assessment. NITBUT is measured as the time between the last blink and the breakup of a reflected image of a target on the tear film. Tear meniscus assessment carries 75% to 90% of the total tear film volume. Thus, it is used to diagnose aqueous tear deficiency. Tear meniscus parameters used for tear film volume are tear meniscus height TMH (the commonest) and tear meniscus radius of curvature. TMH is measured from the eye lid to the top of the meniscus; the cut-off value is < 0.1mm [15].
Our study aimed to investigate the effects of different types of smoking on the precorneal tear film stability and quality using questionnaire, clinical tests and non invasive keratography. And to provide community awareness about the dangers of dry eye and its connection to smoking, in addition to ensure community education that passive smoking affects children and causes great problems, as dry eye syndrome.
Materials and methods:
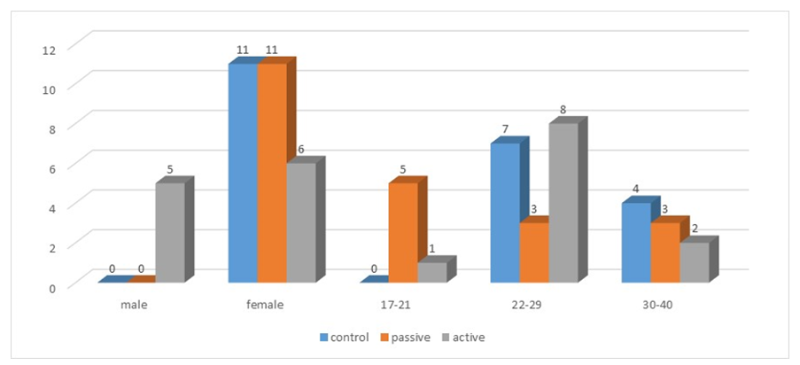
A total of 33 participants were enrolled in this cross sectional study. Informed consent was obtained from all subjects. The participants' ages ranged between 17 to 40 years old with an average 26.5±5.8 years. They were divided into 3 groups: Control non smokers that had no smokers in their family or among their close friends (N = 11), Passive smokers (N = 11), and Active smokers where 82% have smoked for less than 10 years and 18 % are smokers more than 10 years, all participants consumed more than 20 cigarettes during the last period (N = 11). All participants of control and passive smokers groups are females (100.0%), while there are (45.5%) male participants in active smokers group and (54.5%) females (figure 1). there were no statistically significant differences between groups (non – passive - active) smokers related to spherical equivalent; p value was (0.403). All subjects replied to questionnaire for dryness evaluation then went through full ophthalmological examinations. Also we examined subjects for dryness using clinical tests (Tear break up time and Schirmer) and non-invasive keratography.
Subjects with ocular allergic disease, keratitis, ocular surface disease, contact lens wear, glaucoma, previous ocular surgery or injury or subjects with systemic or ocular treatment were excluded from this study. All participants of the present study were asked to stop any treatment with systemic or local anti-inflammatory or anti-histaminic drugs as well as any treatments such as antibiotics, NSAIDs and corticoids prior to their first visit to our clinic.
Statistical Analysis:
The data were analyzed using Statistical Package for Social Sciences program (SPSS) version 25.0. Ophthalmic parameters were collected from both eyes (OU) of all participants. Normality was checked using Kolmogorov-Smirnov normality test. ONE WAY ANOVA was used to compare the means of percentage of contribution of teat film stability between 3 groups. An independent sample t test was used to examine possible relationships between clinical and non invasive keratograph TBUT in each group. P-value < 0.05 was considered statistically significant.
Figure 1: Age and sex distribution in three studied groups
Results
Dry Eye Questionnaire analysis [16]:
Table (1) showed dry eye questionnaire score analysis among studied groups: all control group participants were normal and not dry. There were [6] participants of passive smokers group with percentage of (54.5%) dry, while [5] participants with percentage of (45.5%) were not. And [6] participants of active smokers group with percentage of (54.5%) were dry, while [5] participants with percentage of (45.5%) were not dry.
|
Groups |
|
Total |
||
|
dry |
Not dry |
|||
|
control |
F |
0 |
11 |
11 |
|
% |
0.0% |
100.0% |
100.0% |
|
|
passive smokers |
F |
6 |
5 |
11 |
|
% |
54.5% |
45.5% |
100.0% |
|
|
active smokers |
F |
6 |
5 |
11 |
|
% |
54.5% |
45.5% |
100.0% |
|
|
Total |
F |
17 |
16 |
33 |
|
% |
51.5% |
48.5% |
100.0% |
|
Table (1): Dry Eye questionnaire DEQ analysis among studied groups
Descriptive analysis of measured dry eye ophthalmic parameters:
Table 2 revealed clinical dryness tests measured (TBUT & Schirmer test) among the three studied groups, where TBUT values were (8.41 ± 2.91,7.64 ± 3.14, and 10.31±2.52) in control, passive and active smoker groups respectively. As regards Schirmer test, values were (14.75 ± 8.76, 14.295 ± 10.82, and 14.49 ± 7.765) in control, passive and active smoker groups respectively. By using One-way Anova test to compare clinical data we concluded that there were no statistically significant differences between groups related to TBUT & Schirmer tests, where p value was 0.095, 0.993 respectively. The previous result indicates that there is convergence in the results of the three groups for these tests.
 Table 2: Changes in clinical TBUT and Schirmer test in each group (One-way Anova)
Table 2: Changes in clinical TBUT and Schirmer test in each group (One-way Anova)
Table 3 demonstrated noninvasive keratograph dryness tests measured (TBUT & TMH) among the three studied groups, where TBUT values were (8.26 ± 2.82, 6.31 ± 1.97, and 9.54 ± 3.03) in control, passive and active smoker groups respectively. As regards TMH test, values were (0.41 ± 0.19, 0.42 ± 0.19, and 0.32 ± 0.07) in control, passive and active smoker groups respectively. By using One-way Anova test to compare data we concluded that there were statistically significant differences between groups related to TBUT where p value was 0.026, with insignificant reduction of TMH values in active smoker group as P value was 0.283.
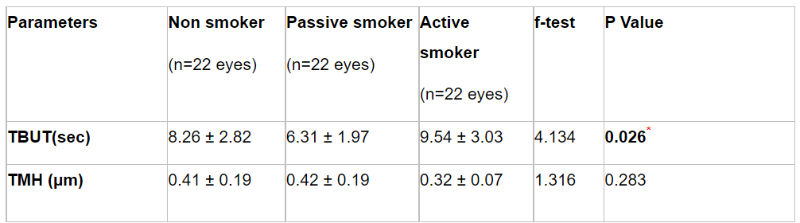 Table 3: Changes in TBUT and TMH tests using non invasive keratograph in each group (One-way Anova)
Table 3: Changes in TBUT and TMH tests using non invasive keratograph in each group (One-way Anova)
|
Group |
Non smoker
|
Passive smoker
|
Active smoker
|
|
Clinical |
8.41 ± 2.90 |
7.63 ± 3.15 |
10.32 ± 2.52 |
|
Non invasive |
8.25 ± 2.82 |
6.31 ± 1.97 |
9.54 ± 3.03 |
|
t-test |
0.165 |
1.645 |
1.341 |
|
p. value |
0.872 |
0.131 |
0.210 |
Table 4: Correlation between clinical and non invasive keratograph TBUT using independent sample t-test test
Table 4 appraised that, there were no statistically significant differences between groups (non passive - active) smokers related to TBUT measured clinically and by using Non invasive Keratograph where p value were (0.872, 0.131, and 0.21) respectively. So clinical test and non invasive keratograph are both correlated in measuring TBUT and can be used interchangeably in the clinic.
Discussion:
Most studies support the hypothesis that smoking is harmful to ocular health and detrimental to the tear film. Active and passive smoking have been proven to reduce tear film stability and quality [17].
This study highlighted a statistically insignificant reduction in clinical TBUT test values in subjects exposed either to active or passive smoking. This was in agreement with Muhafiz et al., 2019 &A˘gın et al., 2020 who reported similar values in smokers and non smokers [18-19]. But it was inconsistent with Matsumoto et al., 2008[20]; Thomas, 2012[21]; Sayin et al., 2014[22]; Aktas¸ et al., 2017[17]; Acar et al., 2017[23] & Mohidin and Jaafar, 2020[24] who assumed statistically significant reduction in TBUT values in smokers.
Schirmer’s test results in smokers remain in general highly variable and contradictory (Clinch, 1983& Cho and Yap, 1993) [25-26].
Our study reported that Schirmer’s test results were insignificantly lower in the smoking group. This was confident with Altinors et al., 2006 [27]; Matsumoto et al., 2008[20]; Thomas, 2012[21] ; Aktas¸ et al. ,2017[17]; Acar et al., 2017[23] ; Muhafiz et al., 2019 [18] & A˘gın et al., 2020[19] who appraised insignificant difference between smoking and non-smoking groups. On the other hand, it was conflicting with Yoon et al., 2005[28]; Sayin et al., 2014[22] & Khalil et al., 2018[29] who demonstrated a significant reduction in Schirmer’s test values in smokers.
So, in order to prevent or avoid the negative effects of smoking on the tear film the best approach could be a cessation, or at least a reduction, in smoking. If this is not possible, several options to manage dry eye would be used [30]. Prescribing artificial tear substitutes to increase the volume or to stabilize the tear film might be beneficial for smokers to avoid the ocular surface damage. Finally, antioxidant eye drops to prevent oxidative stress of smoking could be considered, as well as lipid containing eye drops or sprays to directly impact the damage of the lipid layer caused by oxidative attack of free radicals and oxidizing molecules carried by smoking.
Conclusion:
These findings suggest that chronic smoking has a negative effect on the ocular surface and affects some tear characteristics. And the chronic ocular irritative effects of cigarette smoking may lead to defects in ocular surface defense.
Future recommendations:
Future studies are recommended to re-evaluate the results with larger sample size and to investigate the correlation between the number of cigarettes smoked per day and the reduction of TBUT and Schirmer’s values.
Conflicts /Competing interests: The authors state no possible conflicts of interest with respect to the authorship, or publication of this article.