Journal of Microbiology and Biochemistry
OPEN ACCESS | Volume 1 - Issue 1 - 2023
ISSN No: - | Journal DOI: 10.61148/MBB
Aradhna Gupta and Bechan Sharma*
Department of Biochemistry, University of Allahabad, India.
*Corresponding author: Bechan Sharma, Department of Biochemistry, University of Allahabad, India.
Received Date: January 08, 2024
Accepted Date: January 12, 2024
Published Date: January 18, 2024
Citation: Sharma B, Gupta A, (2024). “A Review on Energy Metabolism by Neurons”. Molecular Biology and Biochemistry, 12(1); DOI: /MBB/005.
Copyright: © 2024 Bechan Sharma, This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Central control of metabolism means intricate control and coordination of biochemical and molecular pathways that occurs within an organism to maintain the overall well-being of an organism. Several components are involved in this. (i) Hypothalamus can sense nutrient levels, hormones and signals related to hunger and satiety. It regulates appetite, energy expenditure and body temperature. (ii) Hormones like insulin, glucagon regulates glucose metabolism, energy storage and utilization of lipids and carbohydrates. Leptins produced by adipocytes signals brain to reduce appetite when fat stores are surplus while ghrelin secreted by stomach stimulates hunger. Adipose tissue stores energy in form of triglycerides and its metabolism releases energy in form of fatty acid when required. Circadian rhythms like sleep-wake cycle, hormone secretion, body temperature all are controlled by suprachiasmatic nucleus in brain. In this review article we have discussed how different factors like hormones, biomolecules, stress, circadian rhythms affect neurons energy homeostasis.
Agrp; POMC; arcuate nucleus; glucose; lipids; ROS; circadian rhythms; hormones; suprachiasmatic nucleus; leptin; ghrelin
Introduction:
All the systemic metabolic activities of brain are controlled by sympathetic and parasympathetic neurons [1]. The neurons of arcuate nucleus of hypothalamus were the principal commanding centre to regulate different types of metabolism. The other linked controlling centres to it were ventromedial, lateral, dorsomedial and paraventricular nucleus of hypothalamus. Brain stem nucleus like dorsal motor vagal nucleus, parabrachial and solitary tract nucleus, regulate feeding, blood pressure and gastric secretion [2]. The neurons energy expenditure is very high when compared with somatic cells [3, 4]. Brain requires 20% of whole-body oxygen consumption. The energy consumption increases with neurons number and the total energy expenditure in signaling/resting state is constant [5, 6]. The arcuate nucleus has two distinct neurons AgRP/Neuropeptide Y and POMC [7]. AgRP/NPY neurons work along with ghrelin in energy homeostasis [8]. The hypothalamic melanocortin system also regulates energy expenditure and food intake by sensing the metabolic status and accordingly taking action on the information received by the peripheral and CNS [9]. The POMC neurons originate from melanocortin and their ablation/ leptin deficiency causes obesity [10].The food intake and energy expenditure are controlled by both endocrine and neuroendocrine systems [11]. The energy expenditure may be due to muscle overactivity/ shivering or glucose/lipids oxidation in brown adipose tissue. Living beings’ energy is ATP produced by oxidative phosphorylation. The CNS senses energy status by the ratio of [ATP]/[ADP] [Pi] where Pi informs about energy status and value is between 104-105 M-1 by AMPK and any deviation may lead to pathophysiological conditions. Undifferentiated cells can bear the cost of energy changes as each cell is independent whereas for differentiated cell little energy metabolic deviation will make whole organism to suffer [12]. Earlier life-threatening diseases means stroke/ haemorrhage, cancer, organ failures but now under this comes so many diseases that are not very rare like obesity, type 2 diabetes and many more. It was estimated worldwide that about 17% of population is obese and 10% diabetic and obesity is not alone it is always associated with hypertension, type 2 diabetes and stroke [13-15]. Chronic dysregulation in glucose homeostasis leads to obesity/ impairments in memory, cognition due to leptin-insulin insensitivity/lack [16]. High glucose inhibits food intake and low increases [17]. Leptin involves two pathways WNT and PI3K to target brain during glucose lowering or energy expenditure [18]. Excess lipids and ROS generated from fatty acid metabolism in brain have been the cause of hypothalamic inflammation and metabolic dysfunction. Mutation in any of the receptors of insulin, ghrelin, GH, or unrepaired BER all impact the metabolism. In this review article we have discussed how different factors like hormones, biomolecules, stress, circadian rhythms affect neurons energy homeostasis in brain.
Energy metabolism Control
Estrogens:
Estrogens/17β estradiol regulates energy homeostasis in brain [19]. Being anti-inflammatory has direct action on verbal memory or cognition [20]. High estrogens prevent obesity and mutation/ deletion in estrogenic receptor α (Esr 1 gene) in brain is associated with obesity, hypometabolism and hyperphagia [21]. These estrogenic receptors are maximally found in ventromedial nucleus of hypothalamus aiding in energy metabolism by glucose regulation and thermogenesis [22, 23]. Decreased levels promote binge eating causing obesity and inactivating stimulation of brown adipose tissue (BAT) thermogenesis [24]. In women’s decreased level of it is associated with mood lowering/ depression which may be due to decreased amino acid tryptophan [25]. So, it can be said that decreased tryptophan results in decreased estrogen causing depression. Estrogens also helps in transcription of DNA base excision repair and translocation of BER (base excision repair) enzymes between subcellular compartments in brain [26]. The actions of anorexigenic and orexigenic hormones leptin and ghrelin on hypothalamus in maintaining energy metabolism is different. The leptin increases energy expenditure/supress food intake [27, 28] and decreased leptin sensitivity /genetic deficiency of leptin receptors in brain is associated with low levels of estrogen promoting hyperphagia and obesity [29].While ghrelin is known to decrease energy and promotes feeding. AgRP neurons expressed in the neurons of hypothalamic arcuate nucleus are inhibited by leptin and insulin and stimulated by ghrelin [30]. Increased 17 β estradiol on the Pro opio melanocortico neurons (POMC) and AgRP neurons in the arcuate nucleus of hypothalamus (ARC) regulates energy balance in opposite way. POMC neurons stimulates α melanocyte hormone receptors which supresses weight gain and enhanced energy expenditure [31, 32] whereas AgRP commonly known as food driving increases weight and foraging behaviour. Decreased 17β estradiol can be corelated to decreased POMC neurons and increased feeding behaviour. The release of neuropeptide Y (NPY) by AgRP neurons promotes weight gain and feeding [33]. 17 β estradiol decreases the effect of NPY/AgRP resulting in suppressed feeding [34]. Inhibition of serotonin signalling in brain or blockage of estrogen receptors α increases appetite and weight gain [35]. Synergistic effects of cholecystokinin and 17β estradiol triggers anorexigenic behaviour by increasing the expression of c-fos where c-fos is a marker for neuronal activation [36].
Growth hormone (GH):
GH are secreted by the somatotropic cells of anterior pituitary gland, instructs brain to balance metabolism to maintain energy homeostasis [37]. They exhibit pulsatile secretion pattern and are regulated by hypophysiotropic hypothalamic neurons and these neurons express either growth hormone releasing hormone (GHRH), or somatostatin (SST). The SST inhibit GH secretion and GHRH stimulate it [38]. Hormone Ghrelin triggers its release by inducing GH secretagogue receptor [39]. Studies have shown that GH therapy or replacements enhances memory functions in its deficiency/ loss of memory and learning in over secretion [40, 41]. GH in brain show orexigenic effect resulting in hyperphagia and obesity mediated by NPY and AgRP neurons that stimulate feeding [42, 43]. The insulin sensitivity in brain is regulated by GH receptor signalling which is the main cause of memory loss or retention or other neuro diseases as over secretion causes insulin resistance [44]. The insulin levels towards leptin are affected by CNS and ablated GH receptors in leptin receptors expressing cells leads to impaired hepatic insulin sensitivity [45, 46]. Defects in GH or inactivation of its receptors in leptin expressing cells/SF-1 neurons leads to hypoglycaemia and impaired counter regulatory response [47].
Glucose:
Ventral hypothalamus of brain contains arcuate nucleus is the main centre for regulating all types of metabolism like energy and glucose homeostasis etc [48]. Its neuropeptides can stimulate various activities like sensory and effector stimulation of neurons/ activation to inhibition of hormones secretion to target organs, bones formation and remodelling. Its main fuel is glucose and when it runs out of fuel, brain functions like cognition, neurons sensation all are affected resulting in several types of neuro disorders. The insulin independent entry of glucose in brain across the blood brain barrier is by transporters GLUT 1 and 3 in normoglycemic state and insulin dependent are GLUT4 [49-51].For the first time in 1950s it was observed that brain can sense glucose levels [52]. Glucose inhibitory and excitatory neurons are activated sensing glucose levels. In the case of glucose inhibitory neurons, there is increased ATP/AMP ratio by Na-K-ATPase or opening of chloride channels by adenosine mono phosphate activated protein kinase and nitric oxide resulting in hyperpolarization in both cases whereas for glucose excitatory neurons there is increase of ATP/ADP ratio, closure of potassium channels, influx of calcium, depolarization and release of neurotransmitters [53-55]. AgRP and POMC are the two neurons regulating energy conservation and expenditure and studies on genetic ablation and optogenetic activation have indicated that about 40% of AgRP/NPY are inhibitory and POMC are glucose excitatory neurons [56, 57]. Non-neuronal cells like astrocytes (glial cells) maximally present at the interface between blood vessels and neurons have GLUT1 transporters where glucose has 3 fates. It can be oxidized/ stored as glycogen/ converted to lactate by astrocyte neuron shuttle [58].
Ventromedial hypothalamus protects steroidogenic factor 1 neurons (SF-1) which regulates thermogenesis, adiposity, and energy balance. The inhibition of SF-1 neurons will cause impaired or no recovery from insulin induced hypoglycemia due to decreased secretion of hormones glucagon, corticosterone and reduced hepatic glucose production [59]. It has been proved that hypothalamic regions and brain stem in association with peripheral organs are involved in glucose metabolism [60]. Studies have also proved that type 2 diabetes and Alzheimer are interrelated. Increasing age there is glucose intolerance and insulin resistance /insensitivity. Impaired insulin secretion overrules the positive effects of insulin on memory and hyperglycemia resulting in glucose entry in brain. Excess glucose in brain is metabolized by polyol pathway increasing sorbitol and inositol [61, 62]. The increased sorbitol reduces taurine thus affecting cellular osmotic balance. Though glucose is main fuel for basal energy, action potentials, neurotransmitters synthesis [63] it can use other molecules like lactate in exercise, ketone bodies in starvation [64, 65].
Lipids:
Astrocytes supply ketone bodies to neurons when energy is derived from lipids in low glucose intake [66]. During fasting there is increase in ghrelin and accumulation of stearoyl and palmitoyl CoAs in brain which are the leading cause for leptin, insulin insensitivity and hypothalamic inflammation [67-69]. AgRP neurons are stimulated during this period which utilize the extra lipids thus protecting the neurons from lipids toxicity and reducing adiposity/ initiates feeding [70]. Though some studies say that fatty acids have direct and indirect effect on neurons.
Reactive oxygen species (ROS): In fasting’s beta oxidation takes place as an energy source but it also results in increased ROS causing hypothalamic oxidative stress [71].
Malonyl CoA: an enzyme of Fatty acid metabolism has played major role in energy homeostasis in CNS as fatty acid synthesis takes place in surplus energy [72, 73]. Inhibition of Fatty acid synthase in CNS, increases malonyl CoA and decreases food intake resulting in lean phenotype. Overexpression of malonyl CoA decarboxylase removes malonyl CoA resulting in increased food intake and obesity [74].
Bone regulator:
AP1 antagonism studies proved that both neurons (AgRP and POMC) results in glucose consumption, bone formation and reduced adiposity [75]. Though both neurons act as positive regulator of bone formation, AgRP neurons supresses bone resorption whereas POMC neurons increases it. NPY neurons acts as negative regulator of bone formation [76]. Leptin a known adipokine of lipid metabolism is also involved in bone remodelling. It negatively affects bone density via serotonergic and sympathetic nervous systems and positively via a neurotransmitter CART present in POMC and suppression of osteoclastogenesis [77-79]. Thus, it can be said that energy depends on glucose metabolism and bone homeostasis or vice versa bone remodelling can affect energy end glucose levels [80, 81]. Galanin an inhibitory neuropeptide regulates anterior and posterior pituitary hormones which in turn regulates energy and bone homeostasis and its activation depends on different types of food consumption, drugs and alcoholic beverages [82]. The galanin activation cause increase of neurohormones oxytocin, arginine, vasopressin and thyrotropin releasing hormone. Reduced oxytocin leads to obesity and osteopenia [83, 84].
Circadian synchronization:
Metabolic homeostasis and disrupted sleep/wake or feeding /fasting cycle influences circadian rhythms and transcription by interaction with tissue specific metabolic and inflammatory factors like p65, SREPB, PPARα [85,86]. Discovery of immediate early genes in neurons acts as a link between environmental signals and neuronal transcriptions [87]. It can control protein accumulation by regulating translation of processed mRNAs [88]. Studies have proved that release of neurotransmitters like serotonin, dopamine, somatostatin depends on circadian cycles and they respond dynamically inducing transcriptional reprogramming in nutrient changing state across sleep/wake cycle or time of day [89, 90]. The central pacemaker clock in the suprachiasmatic nucleus (SCN) regulates physiology and behavior and its ablation results in loss of rhythm in drinking water and locomotor activity [91]. Vasoactive intestinal peptide active neurons in SCN controls diurnal amplitude in locomotor activity in healthy and Alzheimer patients. Light exposure to SCN in humans and other species during early evening results in delay in walking and sleep onset on the following day [92]. This can be due to rhythmic variations in post synaptic signaling pathways across the light dark cycle and combination of inhibitory-activating signals of glutamate, GABA and PACAP or transmitter/ peptide time scales [93, 94]. The wake promoting orexin expressing neurons in lateral hypothalamic SCN regulates sleep/wake state and shifts across sleep stages [95]. In wakefulness, increased metabolism and energy use by neurons results in accumulation of toxic waste products amyloid and tau proteins [96]. The removal of these waste products from CNS via BBB is thought to be mediated by sleep/wake state and circadian which rely on glial cells [97]. The sleep dependent glymphatic system controls the efflux of metabolites adenosine and toxic proteins like amyloid β and lactate from the brain [98]. AgRP neurons promote wakefulness and POMC sleep [99]. Insulin secretion is regulated by melatonin and melatonin secretion is controlled by SCN. Thus, it can be said that melatonin mediates glucose homeostasis [100, 101].
Conclusion:
We have seen how various components interact to regulate key metabolic processes including energy intake/expenditure, glucose homeostasis, lipids metabolism, circadian rhythms. These pathways are important for proper functioning of neurons as neurons energy demand is very high. Dysfunction in any of these pathways in brain would give rise to different types of diseases like diabetes, obesity, hormonal imbalances, sleep disturbances and metabolic syndromes. The study will be important for developing insights into neurological diseases and potential therapeutic interventions.
Figure 1: How different neurons maintain energy homeostasis
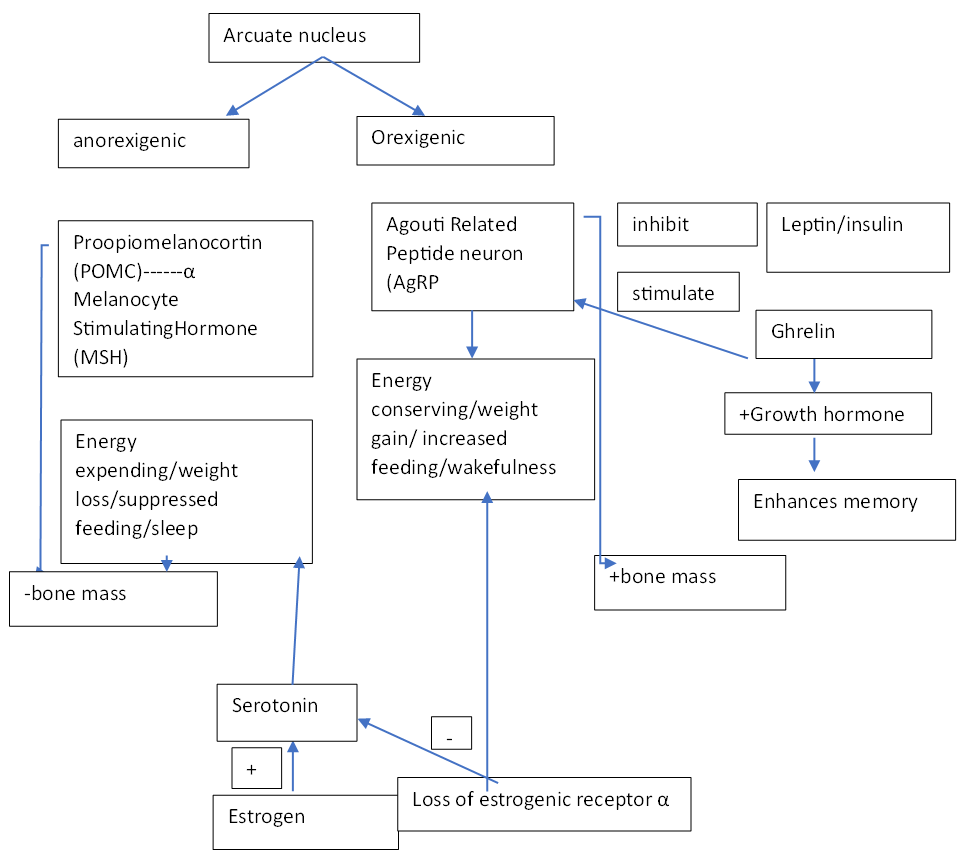
+: increase; -: decrease
Acknowledgments:
The authors are grateful to University of Allahabad for providing facilities for carrying out the present work. AG acknowledges UGC-New Delhi for providing the financial support in the form of a fellowship.
Conflicts of Interest:
There is no conflict of interest to be disclosed.