Maciej Pawlikowski
AGH University of Science and Technology, Department of Geology, Geophysics and Environmental Protection.
Cathedral of Mineralogy, Petrography and Geochemistry al. Mickiewicza 30, 30-059 Kraków.
Corresponding Author: Maciej Pawlikowski, Cathedral of Mineralogy, Petrography and Geochemistry al. Mickiewicza 30, 30-059 Kraków.
Received Date: May 27, 2024
Accepted Date: June 07, 2024
Published Date: June 10, 2024
Citation: Maciej Pawlikowski. (2024) “ Biomineralogy of Atherosclerotic Plaque.”, International Surgery Case Reports, 6(2); DOI: 10.61148/2836-2845/ISCR/073
Copyright: © 2024. Maciej Pawlikowski. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Atherosclerotic plaque is the subject of many different studies (1–68). This article presents mineralogical studies of the plaque from the carotid artery, aorta, coronary arteries, and the mitral and aortic valve leaflets. In the research, we used polarizing microscopy, analytical diffractometry, scanning microscopy combined with an EDS (Energy Dispersive Spectroscopy) analyzer, and more. The study results are presented; manners and causes of the plaque formation are explained, including the components of different types of atherosclerotic plaque. The cause of biomineralization of arteries with the plaque is also presented.
atherosclerotic plaque
Introduction
Atherosclerotic plaque is the subject of many different studies (1–68). This article presents mineralogical studies of the plaque from the carotid artery, aorta, coronary arteries, and the mitral and aortic valve leaflets. In the research, we used polarizing microscopy, analytical diffractometry, scanning microscopy combined with an EDS (Energy Dispersive Spectroscopy) analyzer, and more. The study results are presented; manners and causes of the plaque formation are explained, including the components of different types of atherosclerotic plaque. The cause of biomineralization of arteries with the plaque is also presented.
Material and research methods
Material for this research was provided by the John Paul II Hospital in Krakow, and taken from areas indicated in Fig. 0. Fragments of arteries were stored in buffered formalin. Before the tests, they were washed multiple times with distilled water and ethanol.
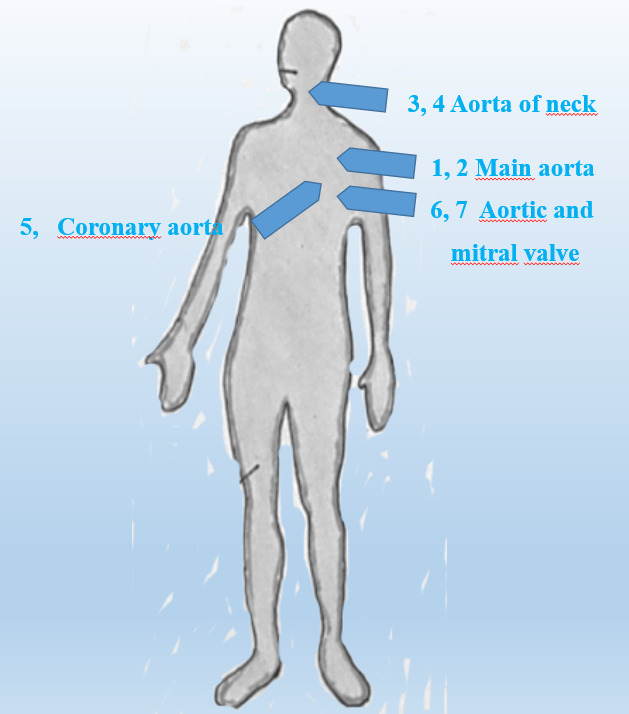
Fig. 0. Sketch showing the arteries involved in the study
Mineral grains isolated from atherosclerotic plaque were prepared for examination under a polarizing microscope by embedding them in epoxy resins. After hardening, they were polished with diamond powder and dried in a vacuum dryer. Then they were examined under a microscope, and the observed phenomena were documented with photomicrographs. A microscope from the Chinese company Meiji was used.
The goal of X-ray diffractometric studies was to identify the phases occurring in atherosclerotic plaque, especially the so-called calcifications. After mechanical separation from the plaque, those were dried and ground in an agate mortar. The powder was analyzed using a Parkin-Elmer diffractometer. Interpretation of the diffractograms was done using the X-rayan computer program.
Observations using a scanning microscope were carried out with the new generation electron microscope FEI QUANTA 200 FEG from an American company. Recognized phenomena were documented with photomicrographs and EDS energy spectra.
The study presents only selected research results.
Research results
AORTAS
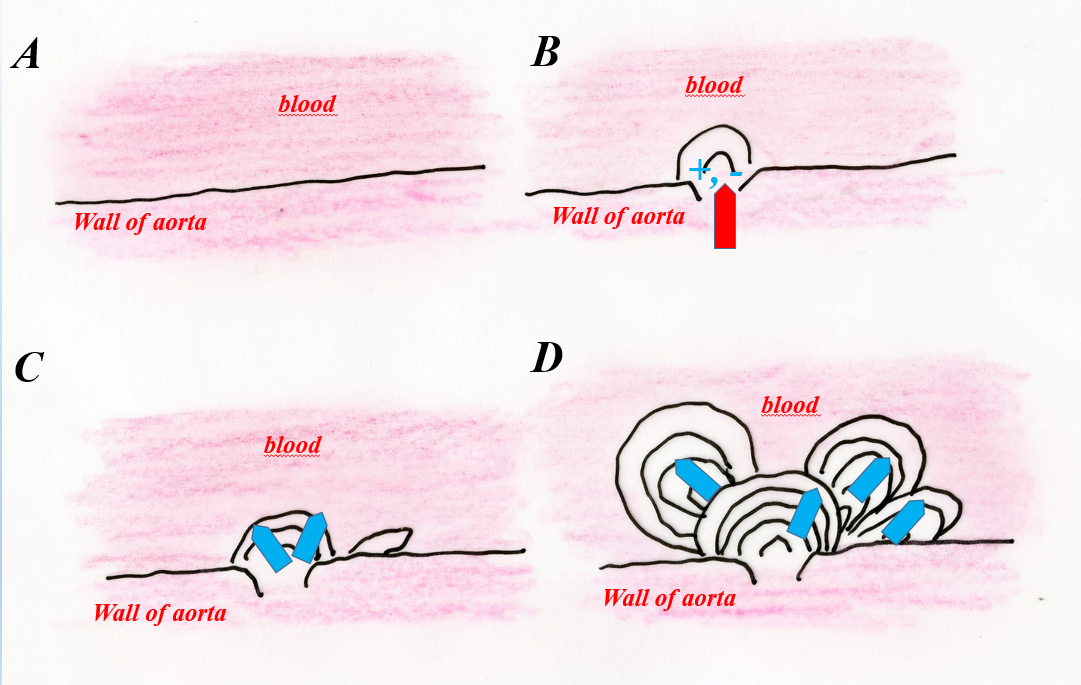
One of the most common forms of atherosclerotic plaque observed in the aorta are aggregated concentrations with a characteristic internal structure (Fig. 1, a, b).
 a
a 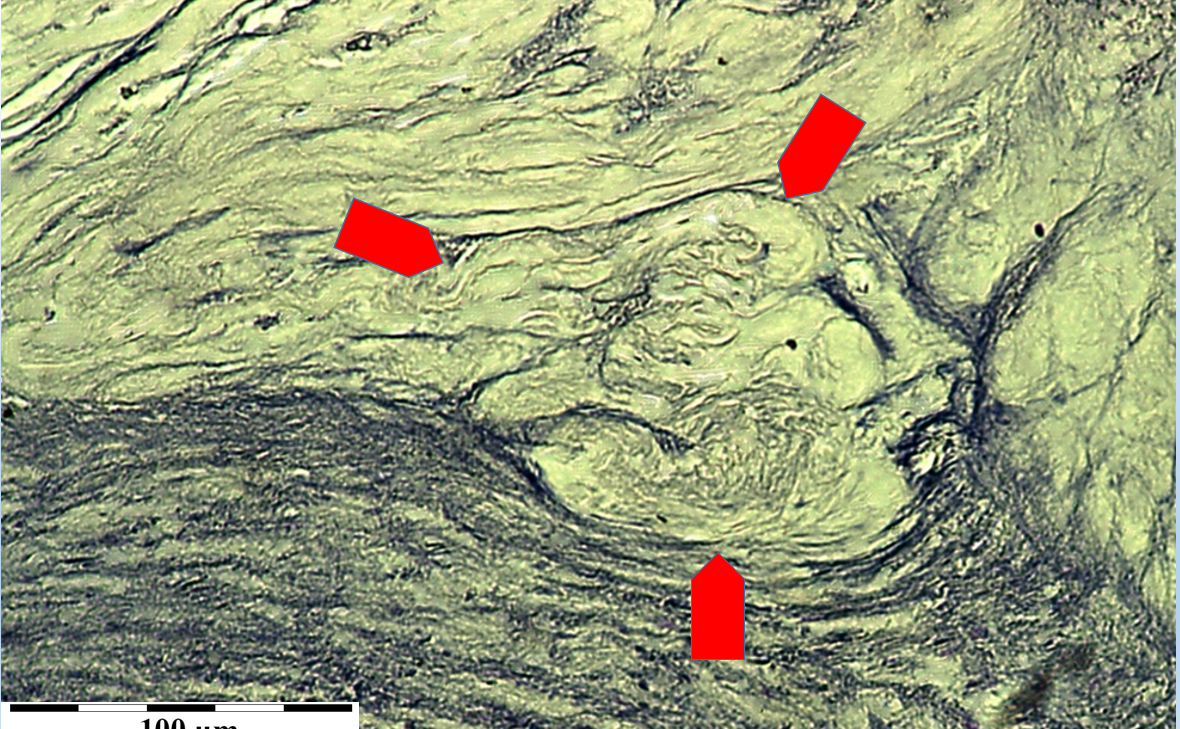
b
Fig. 1. a – diagram showing the development of a fragment of atherosclerotic (cholesterol) plaque. A – phase before plaque formation, B – destruction of the artery wall surface (intima), C – initial phase of cholesterol crystallization in the site of damage to the intima, D – development of aggregate forms of cholesterol concentration.
b – photograph of the internal structure of cholesterol atherosclerotic plaque on the inner wall of the aorta. Biological microscope.
1. Aorta. Biomineralization of internal surface with cholesterol. Type 2.
Another type of cholesterol biomineralization of the aorta involves the deposition of non-crystalline cholesterol at the site of arterial damage and its subsequent reconstruction into crystalline cholesterol (Fig. 1’, a, b). This phenomenon was confirmed by diffractometric, structural X-ray studies (Fig. 1’’, Tab. 1).
 a
a 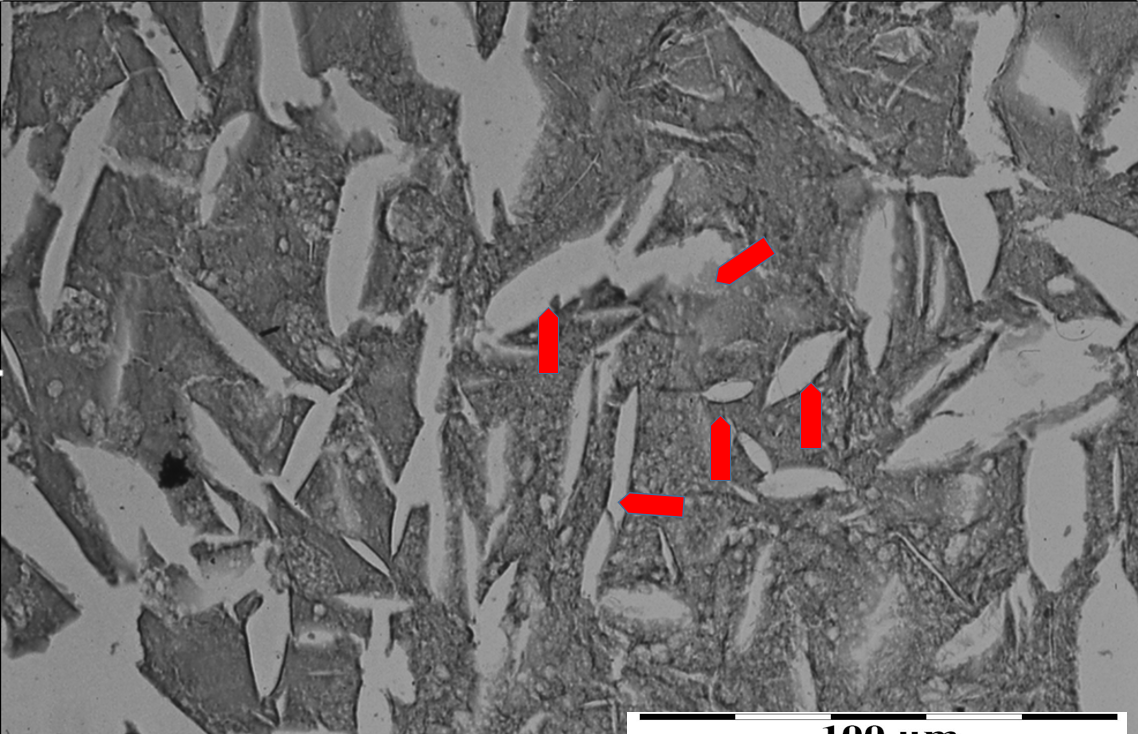
b
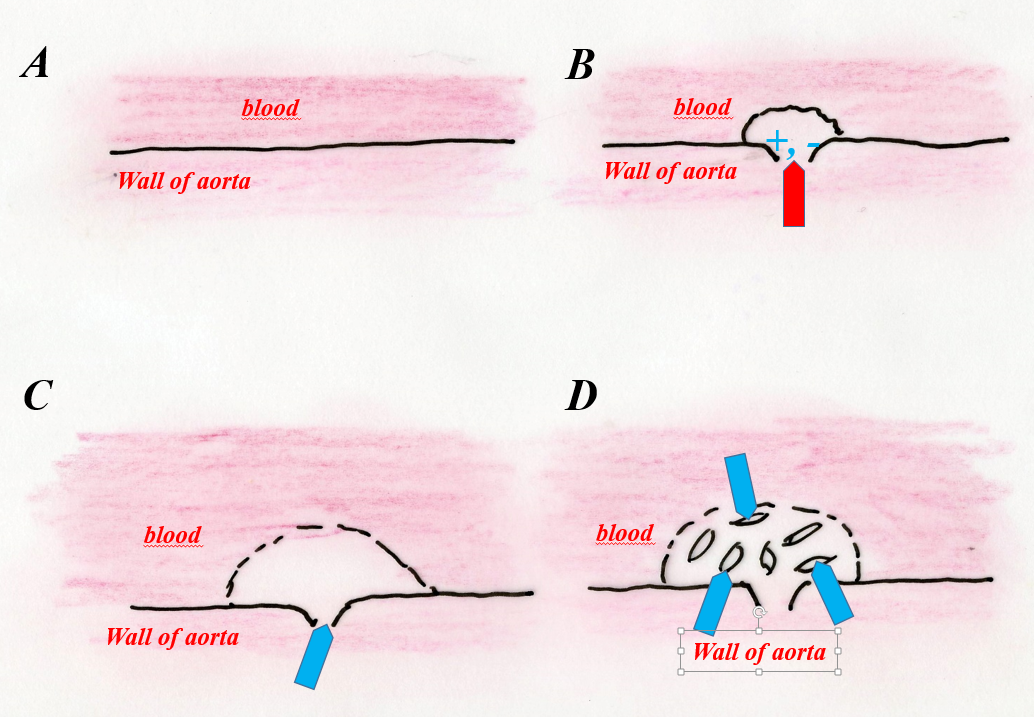
Fig. 1’. a – diagram of the formation of cholesterol plaque on the damaged surface of the intima. A – contact of the artery with blood before the formation of atherosclerotic plaque, B – initial phase of cholesterol plaque formation at the site of damage to the inner wall of the artery (arrow), C – phase of deposition of cholesterol with a disordered atomic structure at the site of arterial wall damage (arrow), D – recrystallization phase (organizing the structure) of non-crystalline cholesterol into crystalline cholesterol (arrows).
b – atherosclerotic plaque formed on the inner wall of the aorta. Boat-shaped cholesterol crystals (arrows) embedded in a mass of cholesterol with a disordered structure. Polarizing microscope, partially X polaroids.
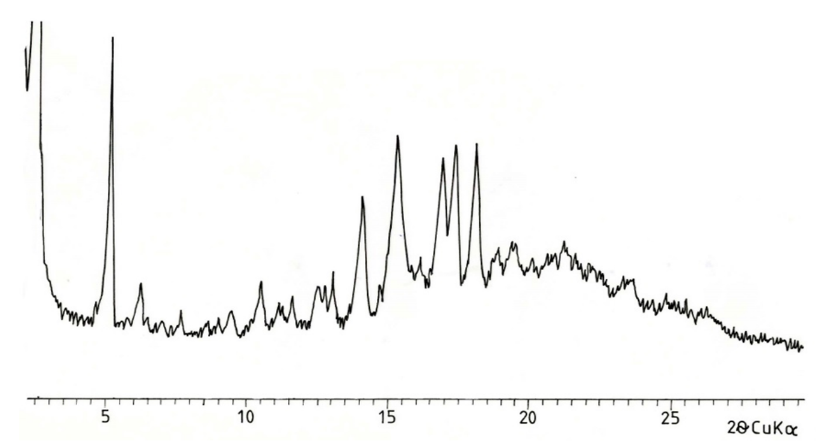 Fig. 1’’. X-ray diffractogram of cholesterol atherosclerotic plaque from the aorta
Fig. 1’’. X-ray diffractogram of cholesterol atherosclerotic plaque from the aorta
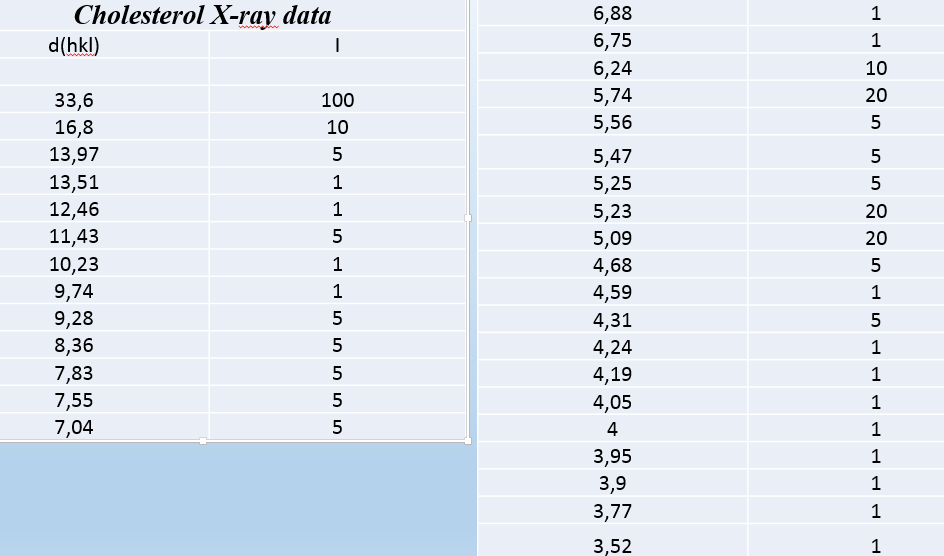
Tabble. 1
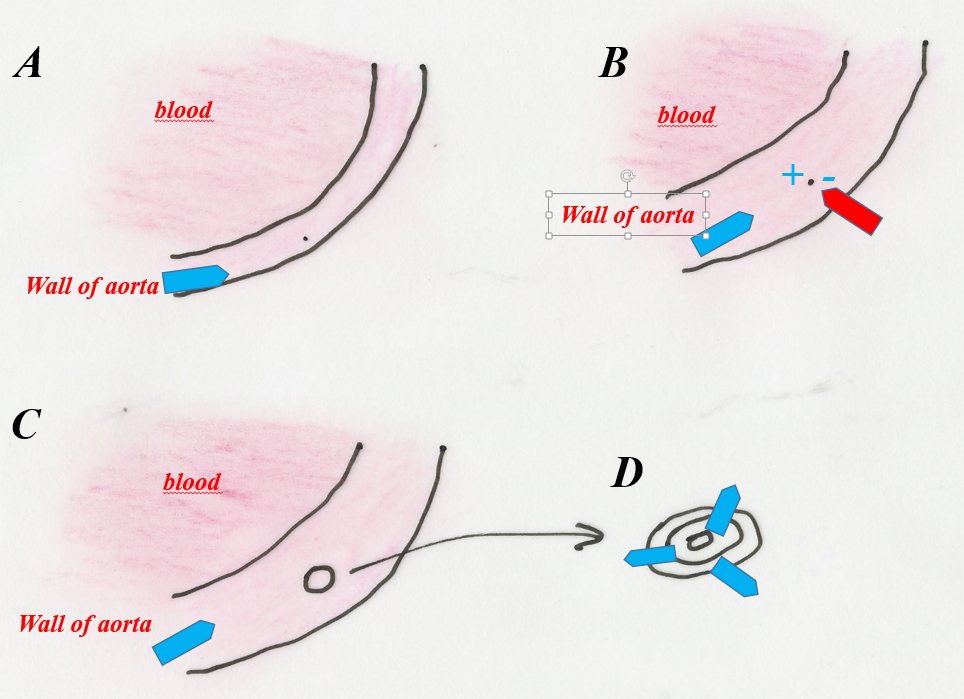
This type of biomineralization is often associated with increased phosphorus and calcium content in arterial blood, which may occur for various reasons. It develops, like other types of arterial biomineralization, in places of artery damage, both on its inner wall and in the wall itself (Fig. 2, 2’).
 a
a 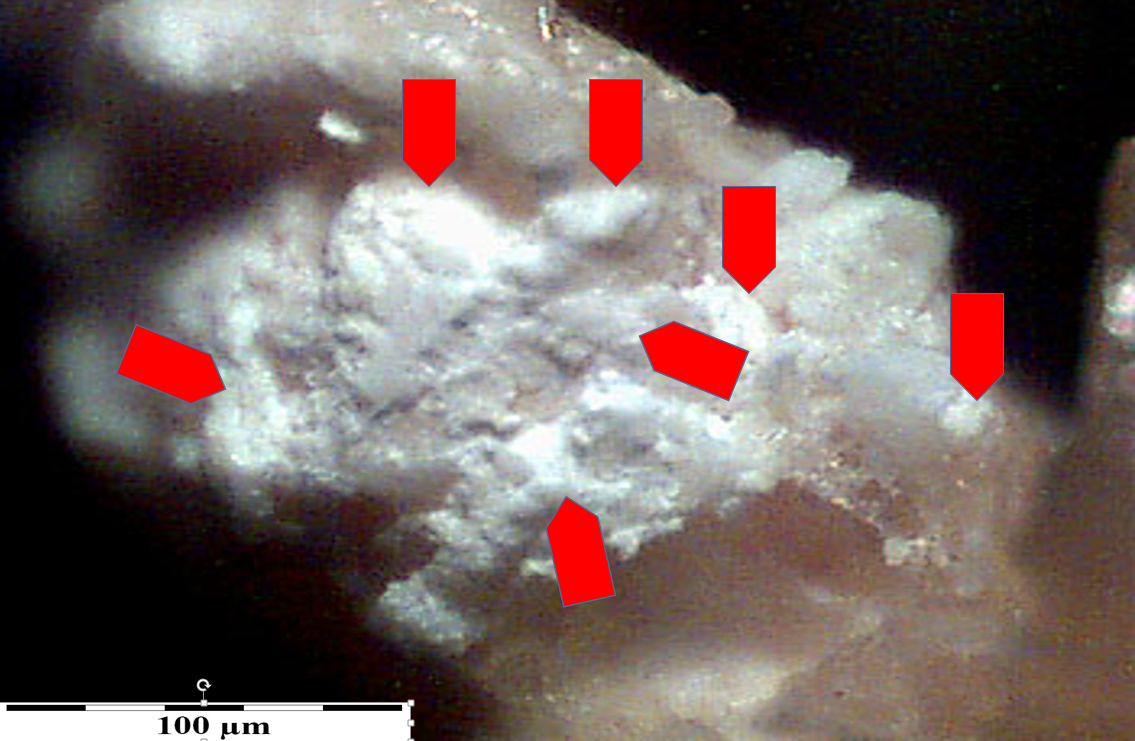
b
Fig. 2. a – phases of biomineralization of the aortic wall by phosphates. A – phase before the initiation of phosphate crystallization, B – phase of destruction of the muscular tissue in the aortic wall, C – phase of attaching phosphate and calcium ions to the damaged structure of the aortic muscle tissue. Formation of a phosphate “grain” composed of hydrated phosphates with a poorly ordered atomic structure, D – growth of the phosphate “grain” and organizing the structure (crystallinity) of phosphates.
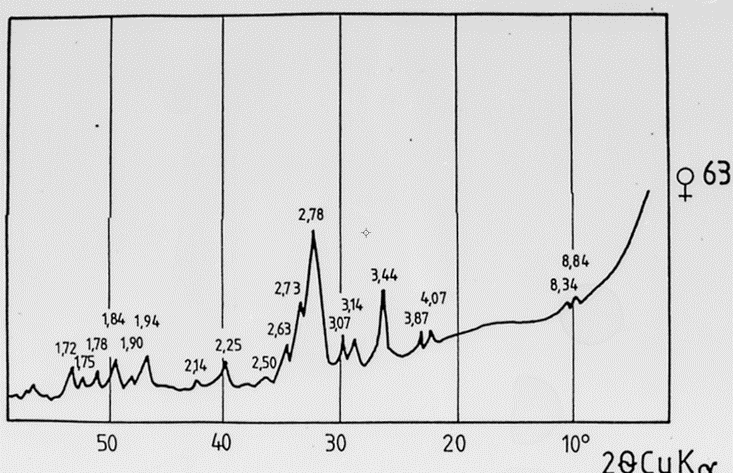
Fig. 2’. X-ray diffractogram of a phosphate grain removed from the aortic wall.
It can form on the artery wall, reducing its diameter and making the transport of blood to the brain and other parts of the head difficult. It develops in places of damage to the artery wall, including the intima (Fig. 3). Fragments of atherosclerotic plaque prepared from this artery and included in the study were sometimes 80% clogged with atherosclerotic plaque.
 a
a 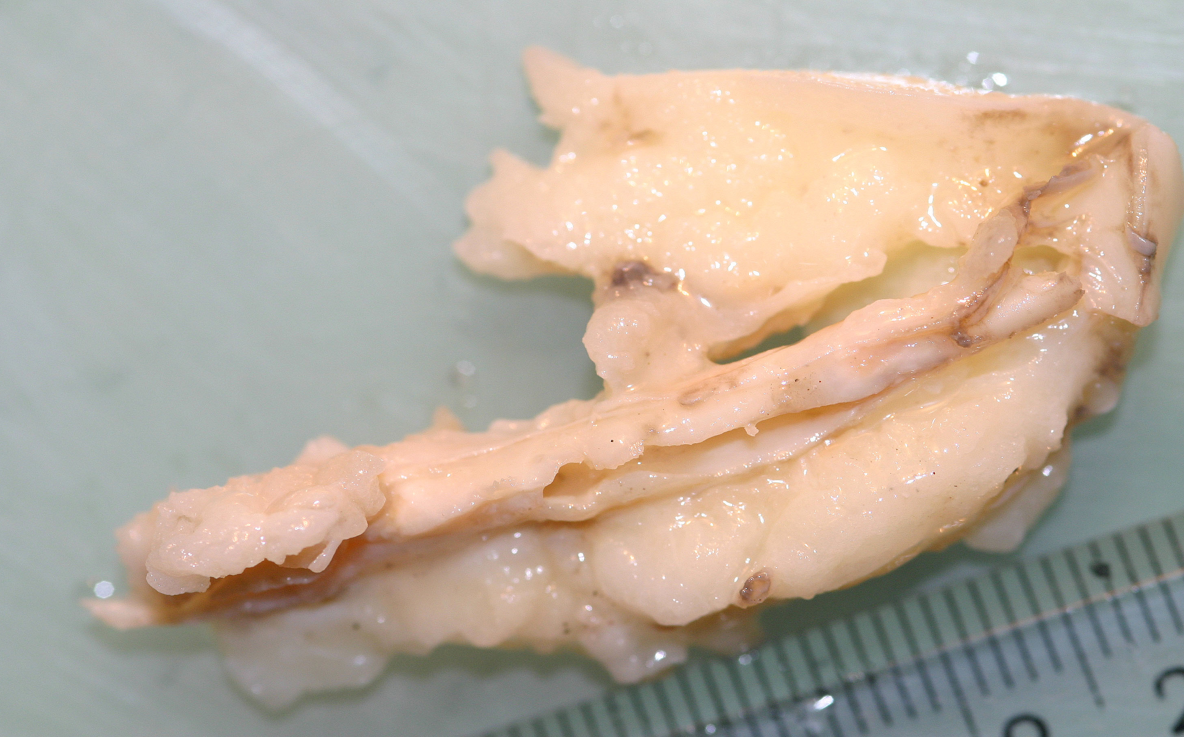
b
Fig. 3. a – phases of development of atherosclerotic plaque on the carotid artery wall. A – phase before plaque formation, B – moment of destruction of the artery wall and creation of an electric field around the damaged area, C – phase of development of atherosclerotic plaque made of cholesterol, D – phase of cholesterol recrystallization into crystals.
B – fragment of the examined carotid artery, heavily overgrown with atherosclerotic plaque.
In small atherosclerotic plaques, cholesterol is poorly crystalline. In larger plaques, recrystallization may result in the formation of characteristic crystals (Fig. 3’).

Fig. 3’. Characteristic cholesterol crystals from the surface of atherosclerotic plaque in carotid artery. Scanning microscope.
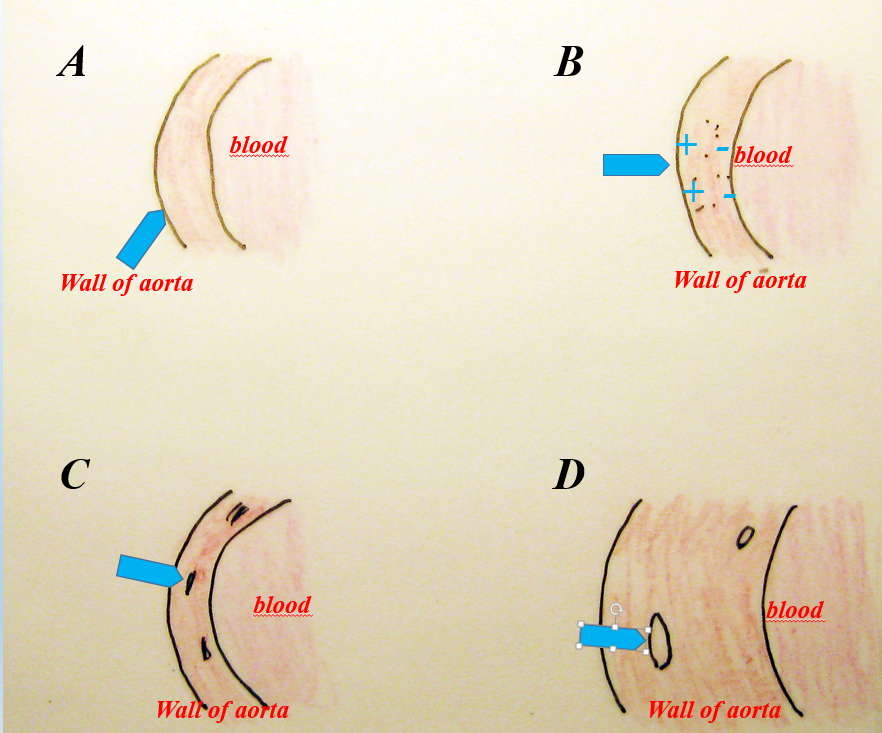
Phosphate biomineralization of the aorta often also develops in its wall, causing its stiffening and deformation. It develops at the site of arterial damage, which can result from various causes (Fig. 4). Often, phosphorus compounds (so-called calcifications) coexist with cholesterol, creating aggregate structures.
 a
a 
b
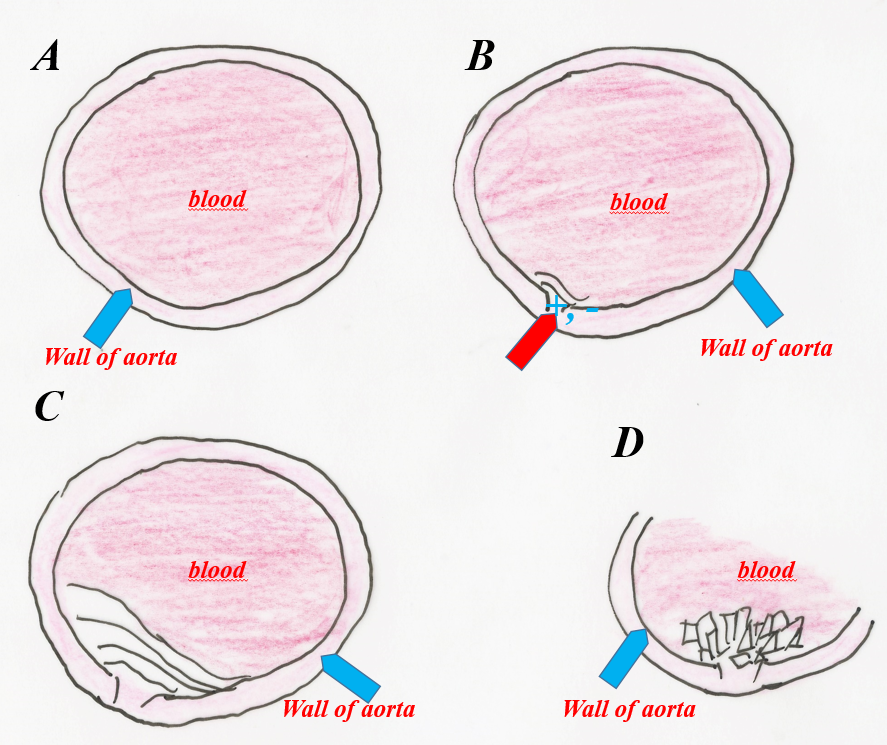
Fig. 4. a – diagram of carotid artery biomineralization. A – phase before biomineralization of the artery wall, B – phase of destruction of biological structures of the wall (breaking interatomic bonds) and creation of a local electric field, C – initial stage of formation of phosphate grains, D – phase of enlargement of phosphate grains with an admixture of cholesterol.
b – microscopic image of the artery wall with concentrations containing phosphates and cholesterol (arrows). Biological microscope, magnification 200x.
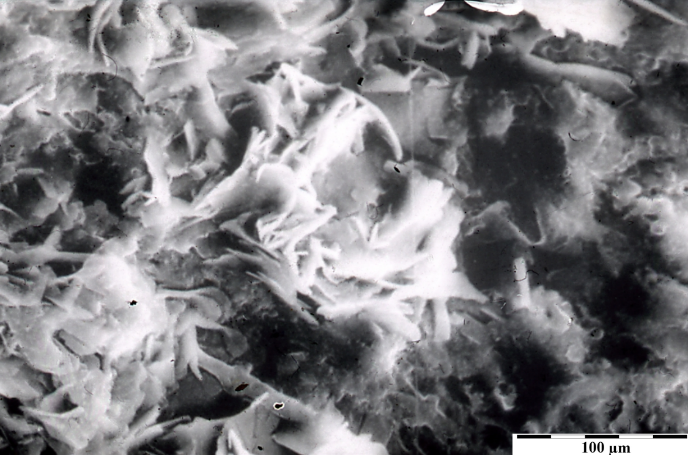 Fig. 4’. Internal structure of the phosphate-cholesterol grain from the carotid artery wall. Scanning microscope.
Fig. 4’. Internal structure of the phosphate-cholesterol grain from the carotid artery wall. Scanning microscope.
5. Coronary arteries. Mixed biomineralization with cholesterol/phosphates.
Biomineralization of the coronary arteries of the heart is of a micro nature. It is recognized in biological, mineralogical, and scanning microscopes only at high magnifications. It affects both the inner surface of the arterial walls and the arterial wall itself (Fig. 5).
a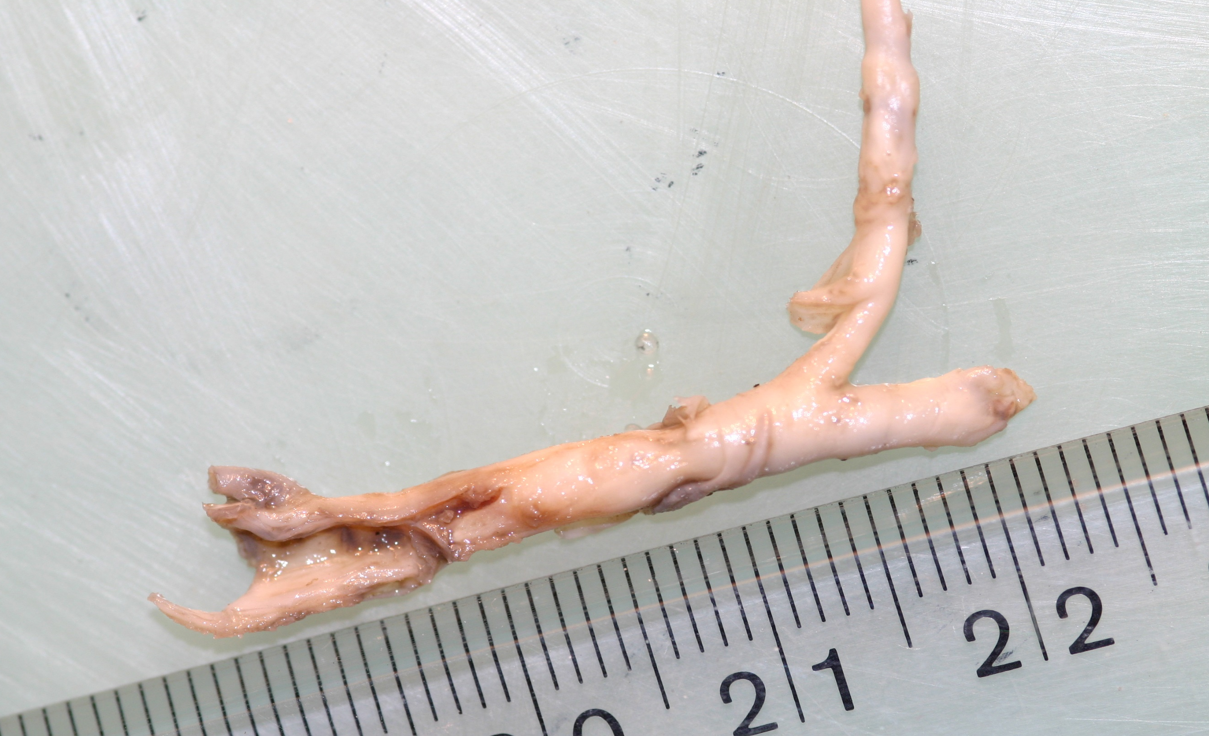 b
b
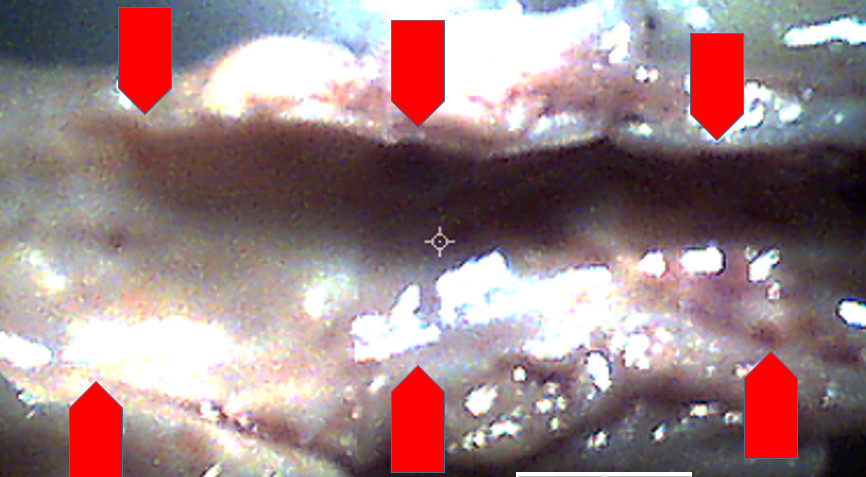
Fig. 5. a – diagram of biomineralization of coronary artery elements. b – fragment of the examined coronary vessel Often, atherosclerotic plaque in coronary arteries has a variable character. After cutting the arteries lengthwise, aggregated clusters are observed (Fig. 5’). In addition to the classic atherosclerotic plaque, grains of white calcium phosphates are sometimes observed on this plaque (Fig. 5’, a).
Observations using a scanning microscope indicate that sometimes the cholesterol present in the plaque is in a microcrystalline form (Fig. 5’, b).
 a
a 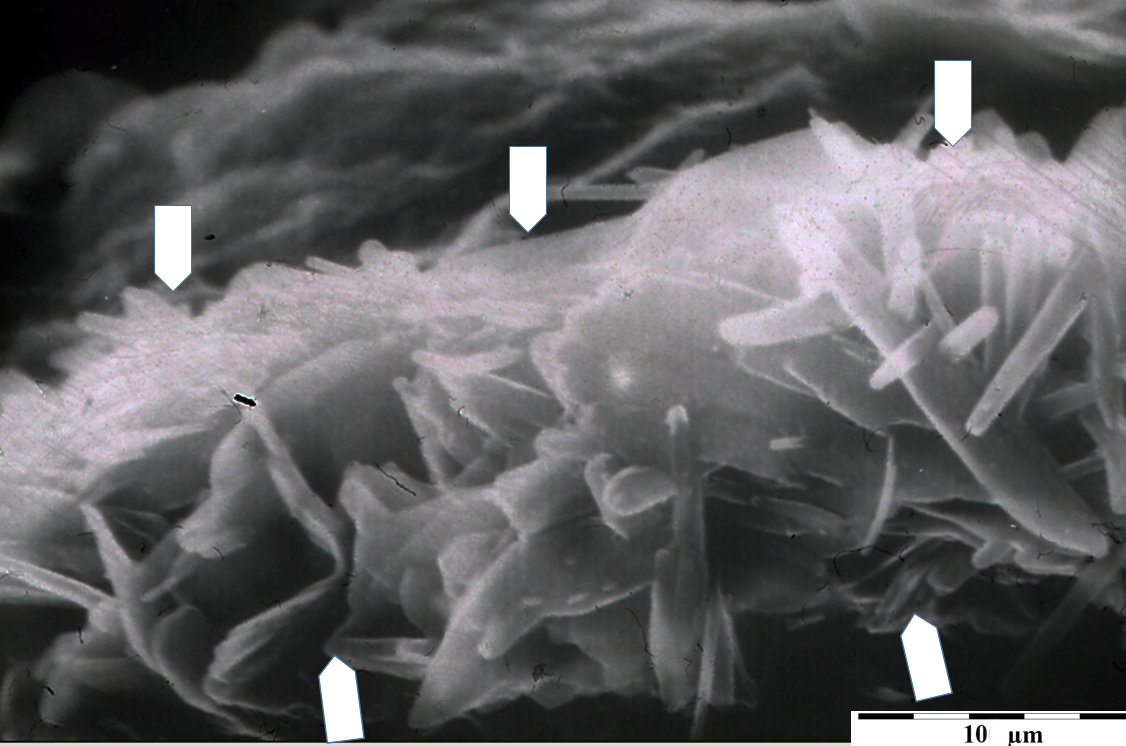
b
Fig. 5’. a – image of a coronary artery cut lengthwise, with visible white phosphate grains crystallized on the cholesterol atherosclerotic plaque (arrows). Digital microscope, 40x magnification.
b – microscopic image of the internal structure of crystalline cholesterol within the atherosclerotic plaque of the coronary artery. Scanning microscope, magnification according to scale.
Heart Valves
The valves become biomineralized because blood flowing through them “hits” their elements with a pressure higher than in the arteries. If arterial blood carries toxins produced by microorganisms infecting our body, the biological structures will be much more damaged in the heart, and especially in the valves. The sites of resulting damage are potential places of biomineralization (Fig. 6).
In mitral valves, aggregated concentrations of calcium phosphates and, slightly less frequently, cholesterol, are observed in their various elements (Fig. 6, b)
 a
a 
b
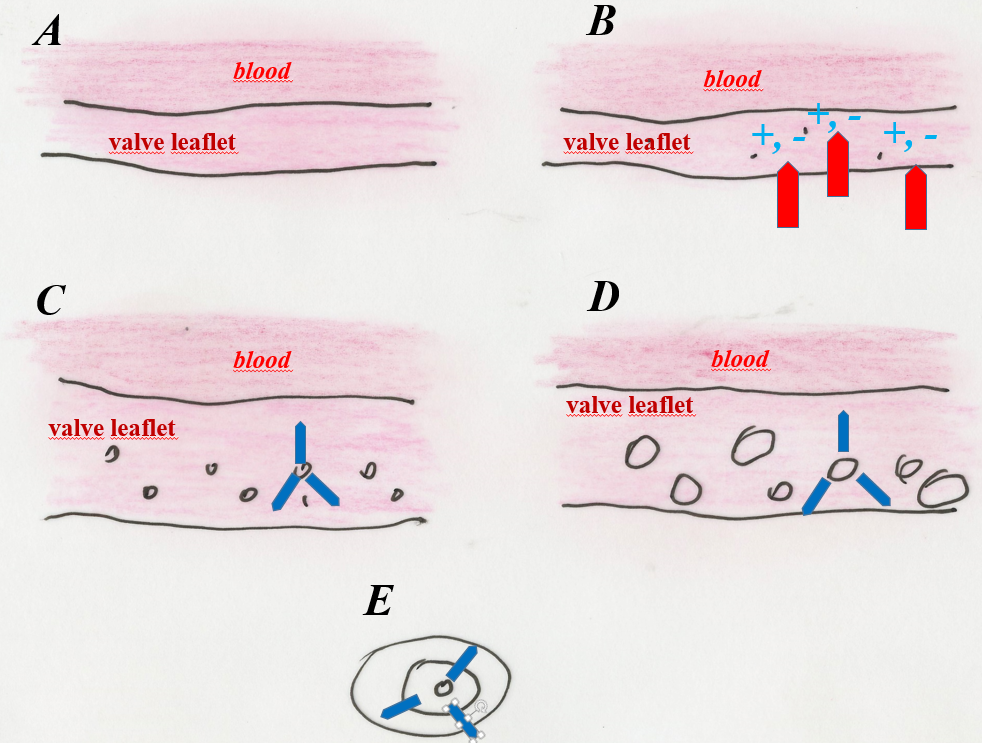
Fig. 6. a – diagram of the formation of calcium phosphate grains in the mitral valve leaflets. A – phase before mineralization, B – formation of zones of destruction of biological structures within the valve elements, C – initial phase of phosphate grains development in the parenchyma of the valve leaflets, D – formation of phosphate grains up to 2–3 mm in size within the parenchyma of the valve leaflets. They practically prevent the valve from working by stiffening it, which causes its regurgitation, E – diagram of the internal structure of a phosphate grain removed from the mitral valve.
b – mitral valve mineralized with calcium phosphate grains (arrows).
In highly mineralized valves, up to dozens of calcium phosphate grains were found, with structures similar to bone apatite (Fig. 6’, a, b).
 a
a 
b
Fig. 6’. Biomineralization of mitral valves. a – grains of calcium phosphate isolated from one valve. b – phosphate (apatite) grains from the mitral valve, enlarged (scale – mm).
Chemical tests of the internal structure of mitral valve grains, performed using an EDS attachment for a scanning microscope, showed their heterogeneous physical and chemical structure. In addition to phosphates with varying degrees of crystallinity, they also contain fatty and cholesterol inclusions. This indicates that blood chemistry varied during grain formation.
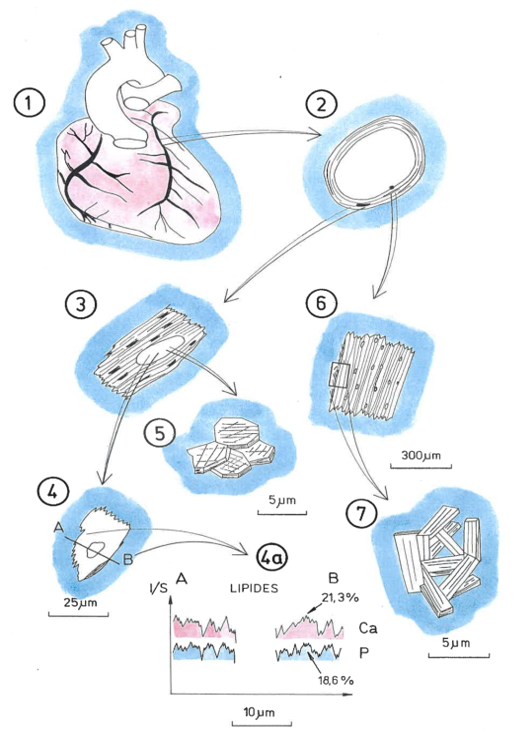
Biomineralization of aortic valves (Fig. 7) and even aortic valve prostheses (Fig. 7’) was observed. It affects both the valve leaflets and the annulus surrounding them. Detailed examinations allowed us to notice that in prosthetic valves (even plastic ones), the beginnings of the biomineralization process are observed at the place where they are sewn into the heart.
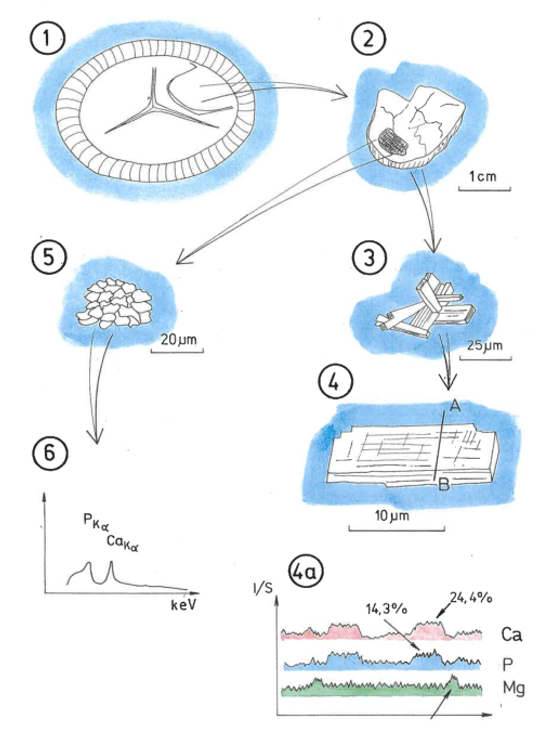
a 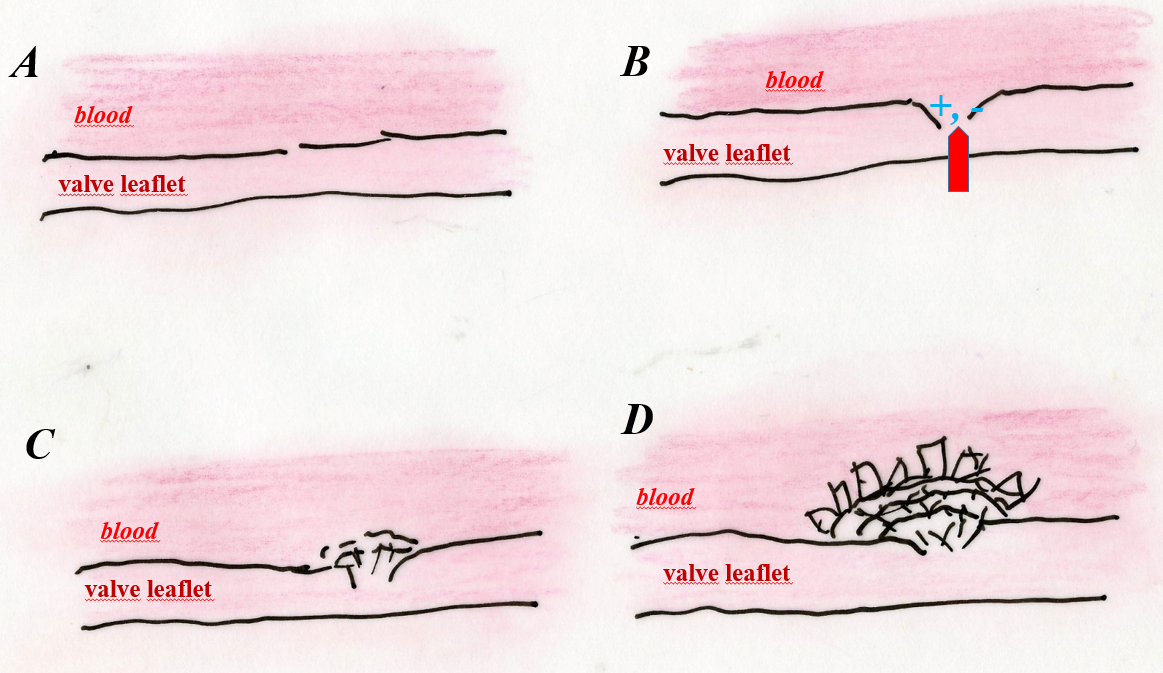 b
b
Fig. 7. a – diagram of aortic valve biomineralization. 1 – the place where the valve leaflet and the annulus meet is covered with phosphate mineralization, 2 – enlarged mineralization zone of the valve leaflet, 3 – internal structure of calcium phosphate crystals from a valve leaflet, 4 – drawing of an enlarged calcium phosphate crystal from a valve leaflet, with a marked line along which its chemical analysis was performed using the EDS method, 4a – curves of Ca, P and Mg content along the line shown on the crystal in 4, 5 – enlarged cholesterol grain embedded in calcium phosphate from the aortic valve leaflet, 6 – EDS energy spectrum of the cholesterol grain shown in 5.
b – diagram of aortic valve biomineralization. A – phase before mineralization, B – damage to the valve leaflet (for various reasons), causing submicroscopic destruction of biological structures (usually collagen fibers that make up the valve), C – initiation of biomineralization of the valve leaflet at the site of its damage, D –phase of development of valve leaflet biomineralization and its stiffening.
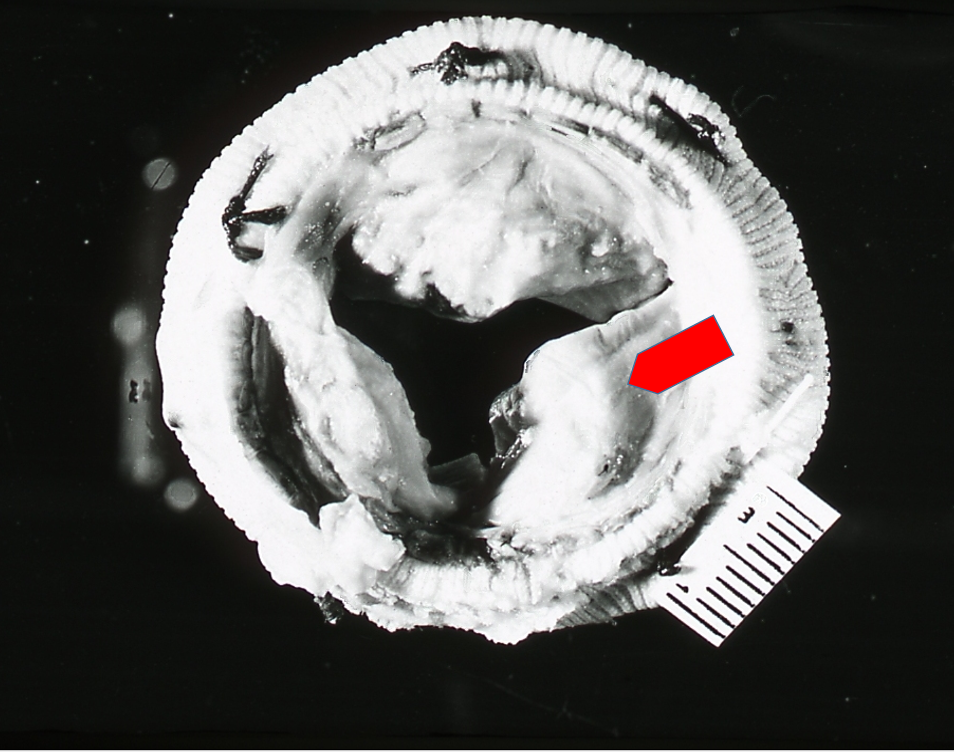
Fig. 7’. A removed aortic valve bioprosthesis with strong phosphate biomineralization (arrow). Visible surgical threads.
Conclusions
Crystallization of atherosclerotic plaque occurs in places where arteries and heart valves are damaged. This applies to both the endothelium and the inner part of the arterial wall.
In places where the artery is damaged, in its various tissues, the interatomic bonds of the biological structures present there are destroyed. The result of breaking bonds is the creation of electric charges in the place of destruction of the biological structure, and the formation of an electric field. This place, from a mineralogical point of view, becomes a crystallization center.
Ions flowing near the damaged tissues of the artery (protoplasts of phosphates, cholesterol, etc.), which are electrically charged, attach to this place and start the crystallization of the atherosclerotic plaque (cholesterol is a crystalline substance).
In the area of tissue damage, so-called ion current is formed, in which cholesterol and phosphate ions, etc. move to the crystallization center, where atherosclerotic plaque is developed (Fig. 8). Ion current is the result of ions being incorporated into atherosclerotic plaque.
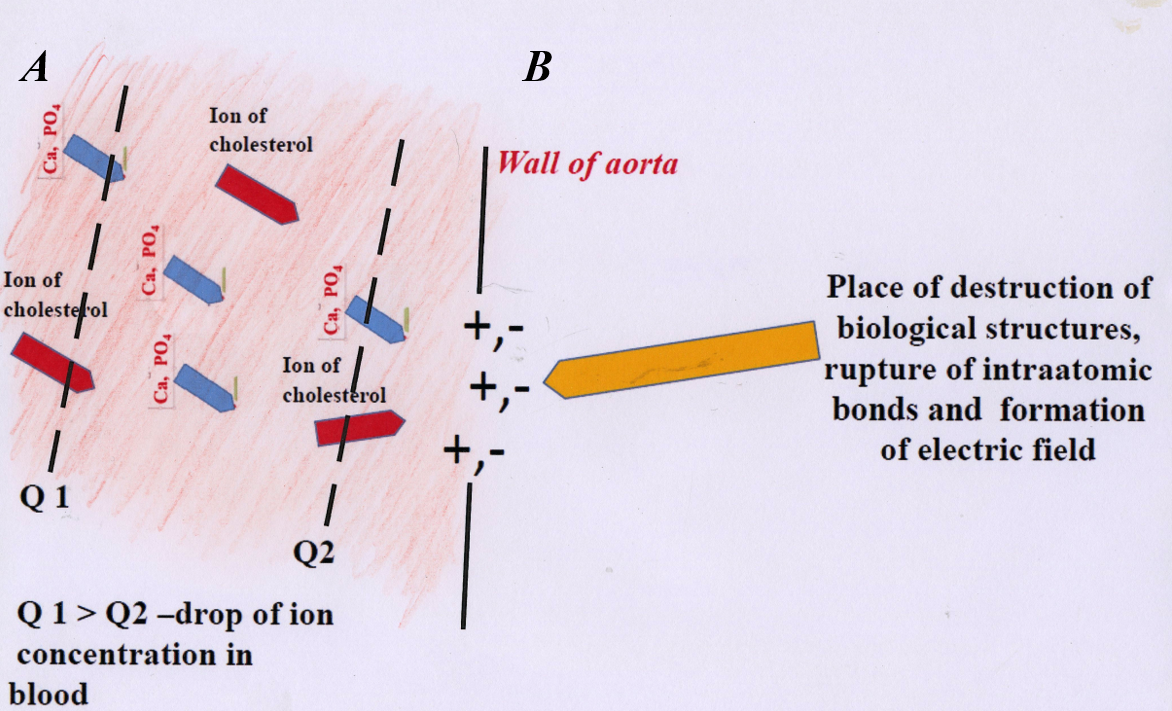 Fig. 8. Diagram of atherosclerotic plaque formation. A – migration of charged ions (cholesterol, calcium, phosphorus, etc.) in arterial blood towards the damaged part. B – if the electric charges are not “saturated” (at the site of artery damage), subsequent layers of “calcifications”, cholesterol, etc. build up on the already formed atherosclerotic plaque.
Fig. 8. Diagram of atherosclerotic plaque formation. A – migration of charged ions (cholesterol, calcium, phosphorus, etc.) in arterial blood towards the damaged part. B – if the electric charges are not “saturated” (at the site of artery damage), subsequent layers of “calcifications”, cholesterol, etc. build up on the already formed atherosclerotic plaque.
Places of destruction of arterial wall structures (crystallization centers) may form for various reasons: genetic or physical (related to mechanical destruction during physical exercise). They are also often the result of past infectious diseases, during which the infecting microorganisms secrete aggressive toxins. Those destroy tissues by causing their lysis (breaking interatomic bonds) in arteries, heart valves and other tissues (e.g. lungs).
Substances that damage arteries and cause the formation of crystallization centers can be solid, volatile, or liquid. These include substances that pollute the broadly understood environment (air, but also drinks, food) that we introduce into the body.
The arteries may be damaged by one of the factors mentioned above or many of them at the same time. Observations indicate that damage formation is largely random.
Therefore, in addition to reducing the level of cholesterol and ionized calcium in the blood, the “fight” against atherosclerosis should focus on preventing the formation of biomineralization centers in the arteries.
A separate problem is related to blocking these centers to prevent the formation of atherosclerotic plaque.
Biomineralization of arteries, i.e. formation of atherosclerotic plaque, does not result from exceeding the so-called solubility product of cholesterol or phosphates, etc. in the blood. It is the result of equalizing the concentration in the blood of the above-mentioned components that build atherosclerotic plaque, and the so-called concentration gradient of ions found in the blood (Fig. 8).
The phenomenon can be compared to the spread of odor in a closed room. If we spray a fragrance in one part of the room, after some time the entire room will smell by equalizing the concentration. When the source of the odor operates continuously and the door to the room is open, the odor will travel towards the open door (in the arteries, this door is the crystallization center).
Depending on what the blood carries, whether it is cholesterol or phosphate ions, the following are formed: phosphate plaque (calcifications), cholesterol plaque (cholesterol deposits), or mixed cholesterol-phosphate plaque with different proportions of both components. This last type of atherosclerotic plaque has been recognized as the most common.
Crystallization of calcifications (phosphates) and cholesterol takes place only in the arteries and elements of the heart pumping the oxygenated blood. The compounds forming atherosclerotic plaque are carried only by oxygenated blood with a pH of just over 7.
Veins transport slightly acidic blood (pH<7), in which both substances cannot crystallize.