International Surgery Case Reports
OPEN ACCESS | Volume 8 - Issue 1 - 2026
ISSN No: 2836-2845 | Journal DOI: 10.61148/2836-2845/ISCR
L.Atroune, K.Bouaita, S. Benallag, N.Habchi, M.Djaafer
Neurosurgery department Mustapha Bacha hospital Algiers Algeria
*Corresponding author: M. Djaafer, Neurosurgery department Mustapha Bacha hospital Algiers Algeria.
Received date: July 16, 2021
Accepted date: July 21, 2021
published date: July 28, 2021
Citation: M. Djaafer, (2021) “Cushing's Disease: Our Experience.”. International Surgery Case Reports, 3(1); DOI: http;//doi.org/03.2021/1.1031.
Copyright: © 2021 M. Djaafer. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Cushing's disease is a potentially fatal disease with serious cognitive consequences affecting the quality of life; its management is multidisciplinary; individualized and complicated and its frequent recurrence is a problem.
Several studies have been carried out to identify the best therapeutic strategy and the biochemical markers that can predict disease recurrence at an early stage. Genetic searches have also been established to find more suitable and precise therapeutic targets, somatic mutations have been reported in P53 and USP8 associated with corticotropic pituitary adenoma.
In our series of 20 cases of cushing's disease out of the 43 surgeries performed with the classic trans-sphenoidal procedure in 13 months, corticotrope adenomas represent 46% of the cases; including a case of recurrence after remission operated in 2009; female to male ratio was 17F / 03M, with an average age of 32 years.
Clinically our patients presented with a facio- troncular obesity, stretch marks, hirsutism in 03 cases, hypertension in 17 cases, and diabetes in 10 cases. Trans-sphenoid surgery was performed for our 20 patients and a disappearance of the rounded face aspect, fat of the cheekbones, weight loss and facio-troncular obesity, we had 04 cases of CSF fistula, 02 cases of transient diabetes insipidus, and 02 cases of cured meningitis: and zero mortality.
Introduction:
Cushing's disease is a serious and rare disease; it is a chronic hypercortisolemia resulting from hypersecretion of cortisol by a pituitary adenoma;
Hypercortisolemia is potentially fatal causing cardiovascular disorders and infectious; while intense hypercortisolemia is a therapeutic emergency;
80% of corticotropic adenomas are microadenomas and sometimes invisible in MRI images; and the treatment of corticotropic adenoma is primarily based on pituitary surgery.
Methods and Materials:
In our series of 20 cases of cushing's disease out of the 43 surgeries performed with the classic trans-sphenoidal procedure in 13 months (January 2011-January 2012), corticotrope adenomas represent 46% of the cases; including a case of recurrence after remission operated in 2009; female to male ratio was 17F / 03M, with an average age of 32 years;
Clinically our patients presented with a facio- troncular obesity ++ (a; b), stretch marks (c; d), hirsutism in 03 cases, hypertension in 17 cases, and diabetes in 10 cases.
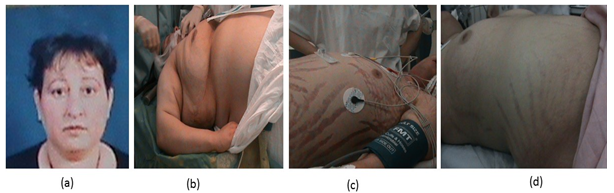
Figure 1: Facio-troncular redistribution of body fat.
Imaging: 17 cases of micro adenomas (grade 0 according to DEROMEOME classification), 02 cases of enclosed macro adenomas without suprasellar expansion (grade 1), and one case of macro-adenoma with suprasellar extension without visual impairment (grade 2);
Trans-sphenoid surgery was performed for our 20 patients.
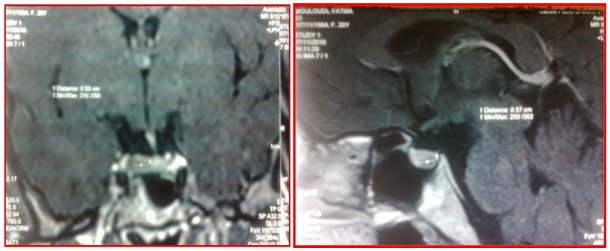
Figure 2: Pituitary MRI showing a microadenoma with deviation of the pituitary stalk (a: coronal plane; b: sagittal plane)

Figure 3: Pituitary MRI evoking a right microadenoma with a well pneumatized sinus.
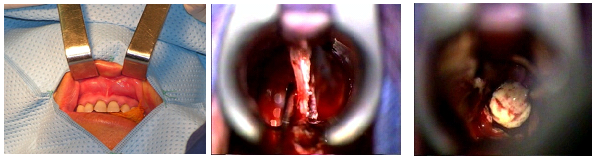
Figure 4: trans-sphenoidal microsurgical approach.
Laboratory results: 16 cases of remission (80%), and 04 cases of failure, including one case with a closed sinus in a woman despite a difficult tumor resection, two cases with very lateralized adenomas in 02 men, and one case with macro-adenoma. Radiological results: Figures 5 and 6
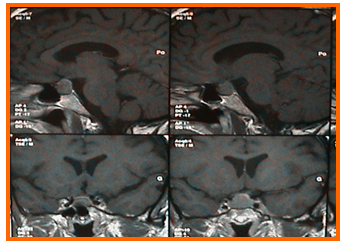
Figure 5: MRI of the brain in sagittal planes (top) and coronal planes (down) showing an enclosed pituitary macro-adenoma.

Figure 6: Postoperative sagittal planes of brain MRI showing an empty sella turcica.
Clinical results:
A disappearance of the rounded face aspect, fat of the cheekbones, loss of
weight and facio-troncular obesity,
we had 04 cases of CSF fistula, 02 cases of transient diabetes insipidus, and 02 cases of
cured meningitis; and zero mortality.
Discussion:
Overt Cushing's Syndrome is a serious condition; it is a corticotropic adenoma leads to chronic hypersecretion of adenocorticotrophin (ACTH), the chronic excess glucocorticoids leads to numerous co-morbidities that affect the quality of life and increase the chances of mortality (1); Cardiovascular diseases and infections are the most common causes of
death (1,2), surgical resection of the causal lesion is the treatment of choice, while we have second-line treatments such as bilateral adrenalectomy, radiotherapy of corticotropic tumors and pharmacological treatments (3).
Curative treatment is not always possible in patients with a non resectable causative tumor or an ACTH-secreting lesion (4);
Recent studies have shown that complications including diabetes, high blood pressure, impaired glucose tolerance, and cognitive deficits may persist after recovery from Cushing's disease (5, 6);
Persistent hypertension after recovery has been reported in 50% of patients previously hypertensive (1,7,8), and 20% of pediatric patients (9); Some studies suggest that transient high blood pressure can persist for 08 months after recovery from the disease (10).
Normalization of cortisone levels is the main treatment, controlling hypercorticism usually improves hypertension but high blood pressure does not always standardized normalize (3);
A key point is currently emerging and that the duration of exposure to excessive levels of cortisone plays a major role in the persistence of co-morbidities and life expectancy after recovery (11), this suggests that the speed of intervention to control hypercortisolemia is of a capital importance.
The treatment of Cushing's disease remains particularly complex and a subject for recent research despite multiple treatments (Figure 7), biochemical control was still no achieved in 30% of patients in a large multicenter cohort (12);
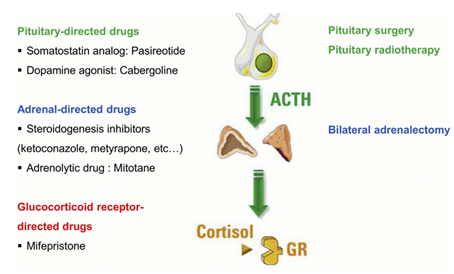
Figure 7: the different therapeutic targets in cushing's disease (3)
Cushing's disease is caused by inappropriate and autonomous secretion of ACTH by a pituitary adenoma, and surgery is the cornerstone of treatment (3);
There is a major point of debate on the surgical indication for non-pituitary adenoma visible on MRI, several groups prefer surgery once the diagnosis is established by ACTH dosage in the inferior petrosal sinus (13,14) basically series have found a reduced cure rate when MRI is negative (14);
Since most adenomas are micro adenomas the ideal surgery is selective adenectomy; in patients whom no adenoma is identified while performing the surgery, a hypophysectomy can be performed with high level of success and low rate of hypopituitarism (3); which is a complication of the surgery.
Partial hypopituitarism occurs in 6.6% and 20.2% of the cases during selective adenectomy and partial hypophysectomy respectively (15).
Other complications: transient postoperative hyponatremia in 5% of the cases (3), CSF fistula, deep vein thrombosis and infections.
Corticotropic insufficiency is the early marker of disease remission while a simple normalization of cortisone values is associated with a risk of recurrence in 10 to 30 % of cases, despite the occurrence of corticotropic insufficiency, patients presented disease recurrence sometimes more than 10 years after successful surgery (16), so the most appropriate term is remission rather than recovery.
The choice of second-line treatment depends on several parameters; Severity of hypercorticism, tumor condition, medical history, concomitant medications, availability and cost of treatment (4);
The success rate after a second surgery may be lower than after initial surgery while the prevalence of complications increases (17);
Radiation therapy has an anti-secretory effect which can occur several months after, it is preceded by an anticortisolic medical treatment, it is often indicated according to oncogenic features of the corticotrophic adenoma, growing macro-adenoma and in the cases of post-operative residue;
Conventional fractionation radiotherapy (CRT) is indicated for patients with large extra sellar, pre-chiasmatic tumors; stereotaxic radiosurgery (SMS) has become the modality of choice for small intra and parasellar tumor.
The indication for radiotherapy should be carefully considered in young women of childbearing age due to the hypopituitarism found in 38.4% for CRT and 29.7% for the SRS (18).
In a recent meta-analysis (19), remission rates after CRT were similar to those obtained by SMS 67.7% / 65.8% with average remission times ranging from months; recurrence occurred in 8.2 and 8.4% of patients after CRT and SRS.
Estrada et al found a remission rate of 83%, 6 to 60 months after CRT in a recent study of 278 patients treated by radiosurgery; a control of hypocortisolemia was obtained in 80% of cases, and a recurrence of 18% was observed in the long term (20).
Before any treatment by surgery or radiotherapy a preparation by a pharmacological treatment
is indicated, even in the event of failure or in patients where surgery can’t be performed, The most common combination used involves various inhibitors of steroidogens (18,21).
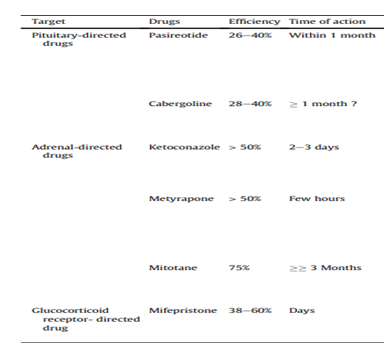
Figure 8: Anticortisolic drugs classified according to their therapeutic target
There are two strategies, one known as the “titration strategy” which adapts the treatement’s dosage to the urinary free cortisol (UFC), the other called "block and replace" associated relatively high doses to block the cortisol with hydrocortisone supplementation;
UFC normalization does not mean restoration of physiological cortisol secretion and a cyclic disturbance of the circadian rhythm of cortisol often persists after UFC normalization, demonstrated by elevated midnight salivary cortisol levels (22);
Recent studies showed that symptoms like weight gain and high blood pressure are controlled in patients with both normalized UFC and midnight cortisol level, than in those with the normal UFC alone.
Long-term use of anticortisolic drugs alone is questionable; most of the authors consider that the anticortisolic drugs should be used as transition treatment before a specific treatment which guarantees the definitive cessation like radiotherapy or bilateral adrenalectomy (BLA).
BLA has significantly reduced the death rate and its efficacy is permanent, its ability to control hypercortisolemia is immediate and uneven; and often considered as a last option (23).
The major drawback is the lifelong supplementation of glucocorticoids, mineralcorticoids and therefore a risk of adrenal crisis;
Improvement in clinical symptoms and quality of life has been reported in majority of patients although there is no comparative study between the results between surgical and pharmacological strategies (24);
BLA is the absolute indication in case of failure of surgical treatment in women desiring pregnancy due to the teratogenicity of anticorticosolic drugs.
The recent discovery of the somatic mutation in the gene for specific ubiquitin protease 8 (USP 8), in almost half of cases of corticotropic adenoma (25) This finding has potential therapeutic implications and warrants further investigations; in fact, during the first months of 2015, two independent investigators reported that USP8 mutations can be seen in one third of adenomas secreting ACTH (26);
Sesta et al established a study of 126 adenomas secreting ACTH, the USP8 mutation identified in 29 cases 23% (27); USP8 is involved in the regulation of the factor epidermal growth rate (EGFR) (28), which in turn plays a role in the development of corticotropic tumors, and involved in the down-regulation of tyrosine kinase activated by the receiver (29);
Tyrosine kinase is a potent mitogen in corticotrope drugs morigenisis (30,31)
Inhibition of ACTH secretion and reduction in tumor size were seen in animal models treated with gefitinib, a tyrosine inhibitor kinase (32), roscovitine is a competitive inhibitor of cyclin-dependent kinases, and was found to have an inhibitory effect on pro-opio-melanocortin in cells of ACTH in xenografted mice (33), roscovitine is currently being clinically tested;
ACTH-MC2R receptor antagonists can reduce cortisol levels without side effects of steroidogenesis inhibitors (3), based on these observations, chimeric molecules that bind to both the somatostatin receptor (SSTR) and the high affinity dopamine D2R receptors are currently being developed (34).
In 2004 WHO defined atypical pituitary adenoma as an invasive tumor with a Ki67 proliferation index greater than 3% and nuclear immunostaining P53 (35,36); Until now the functional and clinical significance of P53 in pituitary adenomas is unclear (37); There are a few published reports on the role of p53 in pituitary adenomas, the gene mutation is rarely detected in pituitary tumors, its analysis was first reported in the work of Herman et al in which none of the 22 pituitary tumors reported had a P53 mutation (38);
Lübke et al studied 19 ACTH-secreting adenomas (10 non-invasive and 9 invasive, reported that none were positive for P53 (39); Levy et al failed to identify a mutation of P53 in 29 pituitary adenomas examined (40);
However in 2009 Kawashima et al described a case of pituitary adenoma in a 63-year-old woman with a P53 mutation (41), Recently, P53 mutations have been obseved in two of six cases of pituitary carcinoma (42).
The present case should continue to be the subject of close observations.
Conclusion:
The management of cushing's disease is multidisciplinary, with intense hypercortilsolimy that’s potentially fatal, and it is a therapeutic emergency; Pituitary surgery remains the first-line treatment, despite the existence of alternative methods.
Due to a high recurrence rate, monitoring is mandatory after remission, it is also important to identify the best monitoring strategy; and define effective therapeutic approaches to reduce comorbidities and improve quality of life of patients.
Pathological and molecular studies have indicated the presence of genetic mutations which can be used as therapeutic targets, currently under development