International Surgery Case Reports
OPEN ACCESS | Volume 8 - Issue 1 - 2026
ISSN No: 2836-2845 | Journal DOI: 10.61148/2836-2845/ISCR
Robin Glorieux 1, Patrick Gillardin 2, Kim Govaerts 3*
1 Trainee in General Surgery at the Catholic University of Leuven
2 Member off Radiology staff at Hospital East-Limburg (ZOL) Genk in Belgium
3 Member of staff Abdominal surgery Hospital East-Limburg (ZOL) Genk Belgium, subspecialized in oncologic surgery
*Correspondence: Kim Govaerts, Department of General and Abdominal surgery (ZOL), Schiepsebos 6, 3600 Genk, Belgium.
Received date: February 07, 2021
Accepted date: February 18, 2021
published date: February 20, 2021
Citation: Glorieux R, Govaerts K, Gillardin P. “Case report with review of literature: Meckel’s Diverticulum with insidious clinical course over 21 years diagnosed on MRI and PET-CT.”. International Surgery Case Reports and Images, 2(1); DOI: http;//doi.org/03.2021/1.1005.
Copyright: © 2021 Kim Govaerts. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
A Meckel’s diverticulum is a rare congenital anomaly of the small bowel. Meckel’s diverticula can remain asymptomatic, however a substantial portion becomes complicated. The pathophysiological features can be subdivided in three groups: obstruction, inflammation and haemorrhage. When symptoms develop the presentation is often acute, especially in case of obstruction and inflammation. However, more insidious clinical courses have been described.
We present the case of a rare insidious clinical course over 21 years of a Meckel’s Diverticulum with intermittent intussusception. PET-CT showed an intraileal polypoid lesion with adjacent small calcifications. MRI showed the same lesion and suggested intussusception. Explorative laparoscopy confirmed the diagnosis and the Meckel’s diverticulum was removed true tangential stapling.
A literature review was performed with discussion of the clinical course, diagnostic work-up and therapeutic management, with a special focus on the radiologic features and the therapeutic challenges in case of intussusception.
Introduction
Meckel’s diverticulum (MD) is a well-known anomaly that originates during the embryological development when the obliteration of the vitelline duct is incomplete. To date, the diagnostic work-up remains challenging. Furthermore, the therapeutic management in day-to-day clinical practice remains subject of much debate. We present a case report of an atypical insidious presentation of MD, including clinical as well as radiological images and a review of literature concerning diagnosis and treatment.
Case report
A 28-year old male patient presented at our polyclinic (Department Abdominal and Oncological surgery in ZOL Genk, Belgium) for a second opinion concerning recurrent episodes of gastrointestinal subobstruction with indefinable radiological features. For this reason, he recently had been hospitalized elsewhere. Plain X-ray and computed tomography (CT) showed intestinal obstruction at ileal level in the right iliac fossa where punctiform hyperdensities resembling operation clips were found (Figure 1 a,b,c).
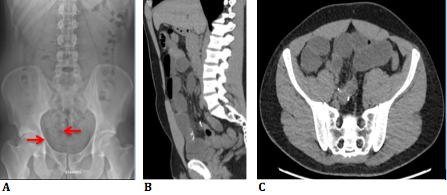
Figure 1a: RX abdomen shows punctiform hyperdensities resembling operation clips.
Figure 1b, c: CT images (sagittal and axial plane) show calcifications and small bowel obstruction.
Interestingly, the patient denied any surgical history. However, another hospital admission for similar subobstructive symptoms seemed to date from 1999 at the age of 7. Reports of CT images with oral and intravenous contrast from that time described a non-specific solid lesion in the pelvis.
At the time of consultation at our centre the patient was asymptomatic and physical examination revealed no abnormalities. We performed a broad diagnostic work-up with FDG-18 Positron Emission Tomography (PET-CT) and magnetic resonance imaging (MRI).
PET-CT showed an intraileal polypoid lesion with light to moderate capitation standardized uptake value (SUV) 5,03 (Figure 2 a,b).
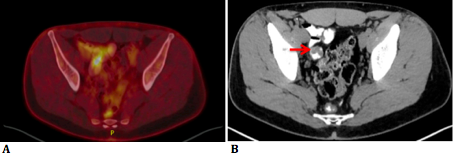
Figure 2a: PET-CT images show an intraluminal polypoid lesion with FDG-19 hypercaptation.
Figure 2b: CT shows intraileal polypoid lesion.
The punctiform hyperdensities described in the previous CT turned out to be small calcifications, presumably originating from calcified lymph nodes near the polypoid lesion. The SUV of the stomach wall, pancreas head and pancreas body were 4.85, 2.64 and 3.66 respectively. It was thought possible that the polypoid lesion originated from a Meckel’s diverticulum. MRI showed the same intraluminal polypoid lesion in the ileum. Intussusception was suggested because of the presence of central mesenteric fat (Figure 3 a,b,c).
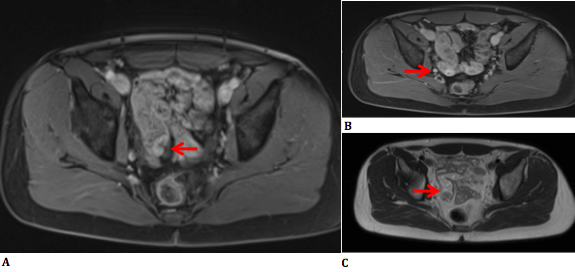
Figure 3a: T1-weighted image (IV contrast) shows central mesenteric fat and a cleft in the lesion suggesting intussusception.
Figure 3b: T1-weighted image (IV contrast) shows moderate contrast hypercaptation in the lesion, except in the centre.
Figure 3c: T2-weighted image.
After IV-contrast administration, moderate enhancement was noted within the lesion; except in the centre where there was hypocaptation, presumably caused by faeces or coagulated blood.
Consequently, an explorative laparoscopy was performed. The diverticulum, which was located at 50 cm from the ileocaecal valve, was divided with an endoscopic stapler device true tangential stapling without narrowing the small bowel lumen. After ex-vivo transection, a polypoid lesion located at least 1 cm from the resection margin at the base was observed (Figure 4).
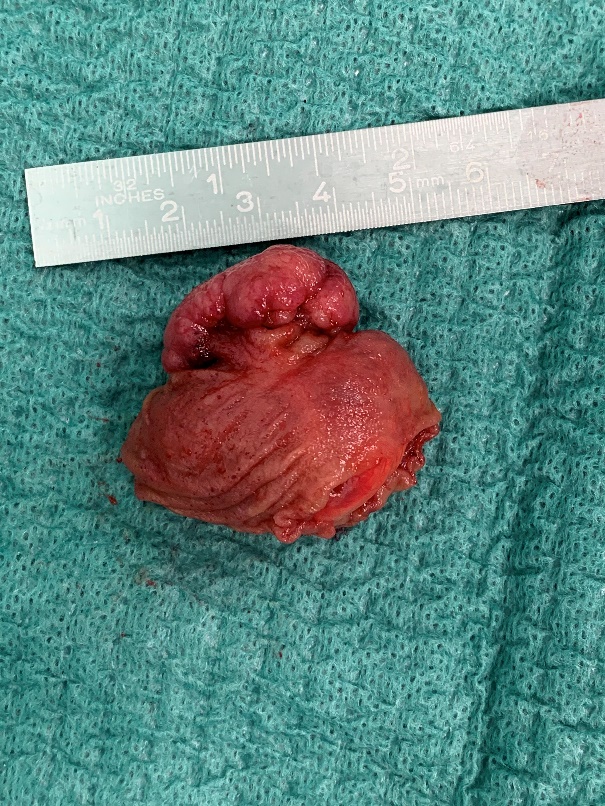
Figure 4: Polypoid lesion after ex-vivo incision of MD: at least 1cm from resection margin.
Histological diagnosis showed a true diverticulum lined by small bowel mucosa, with measurements of 3.5 x 3 x 2.0 cm. The polyp consisted of ectopic gastric and pancreatic mucosa. There was a moderate dense lymphoplasmacytic inflammatory infiltrate in the gastric tissue that resembled a chronic gastritis. No signs of dysplasia or malignancy were found.
The postoperative course was uneventful and the patient was discharged from the hospital the next day. At consultation one-month postoperative the patient remained symptom free and had recovered well.
Discussion
Epidemiology and clinical features
The prevalence of a Meckel’s diverticulum in the general population is estimated between 0,3% and 2,9%, whereas the incidence of symptomatic MD’s is estimated between 4,2% and 9% [1]. In both the adult and paediatric population obstruction has been reported the number one cause of symptoms, respectively 35,6% and 46,7% [1], the main cause of obstruction being intussusception [2,3]. For both paediatric and adult patients gastro-intestinal haemorrhage and inflammation are the second and third most frequently reported complications of MD [1]. Recurrent episodes of gastrointestinal subobstruction with a calcified mass in the right iliac fossa of a male patient can have multiple causes such as appendicular diverticulosis, intestinal tuberculosis, mucocele, sarcoma, lymphangioma, Crohn’s disease… [4,5,6,7]. One of the known clinical features of a MD is intermittent intussusception, however to date this has never been reported together with calcification of the MD itself.
Difficulty to diagnose Meckel’s diverticula has been described before, for example illustrated by a pre-operative diagnosis rate of only 5,7% in a large multi-center retrospective study [8]. If symptoms occur the onset is often acute. However, a more insidious nature with lack of diagnosis for up to 10 years has been reported [9]. Similarly, in our case the insidious and intermittent symptoms contributed to the long clinical course of 21 years. To our knowledge this is the longest clinical course of a symptomatic MD in the available literature.
Imaging
Multiple imaging modalities can be used for the diagnostic work up of MD. Ultrasound has a low cost, is easily accessible, concerns no radiation risk and is therefore frequently used as first and sole investigation. However the accuracy of ultrasound is rather low, with high rates of false negatives [2].
Computed tomography is the most frequently used imaging modality in the acute setting [2]. The available literature concerning the diagnostic accuracy of CT is limited to retrospective studies. Reports of diagnostic accuracy in symptomatic patients ranged between 24% (9/37) and 56,5% (13/23), for asymptomatic patients accuracy was reported at 25,8% (16/62) [10,11]. In general it can be stated that although CT is useful in the acute setting, false negatives often occur and can lead to misdiagnosis.
Our patient had small calcifications around the diverticulum on plain X-ray as well as CT-scan. This is a rare phenomenon and to our knowledge there have been only three other reports of calcified MD’s in the available literature. The first article reports about microscopic calcifications in a MD found during histopathology. The authors made a similar presumption that it was caused by repetitive intussusception [12]. The two other articles reported completely calcified MD’s both authors attributed this to prior acute diverticulitis [13,14].
Another imaging modality is the Technetium-99m scan, also known as “Meckel scan”, which detects MD’s through the accumulation of pertechnate in ectopic gastric mucosa. It has a high sensitivity (89,6%) and specificity (97,1%) in the paediatric population. However, in the adult population sensitivity decreases to 62,5%. Another downside is the risk of false negatives when ectopic gastric tissue is absent or when active bleeding causes dilution of the tracer [1].
18F-FDG PET-CT is an established nuclear imaging technique and is mainly used for the detection and follow up of malignant pathology. Diagnosis of Meckel’s diverticulum without underlying malignancy on PET-CT is rare, to our knowledge there are only two reports in the current available literature. The underlying mechanism of 18F-FDG up-take remains unexplained in the absence of malignancy. However, metabolically active ectopic pancreatic and gastric cells as well as high-density lymphoid follicles of MALT have been suggested [15,16]. In our case ectopic gastric tissue evolving to gastritis could possibly lead to chronic inflammation visualized on PET. But also irritation due recurrent incarceration could be held responsible for the observed FDG avidity. There were no signs of high density MALT. Whether the increased uptake of FDG-18 was caused by ectopic tissue, inflammation or the combination of both remains unclear.
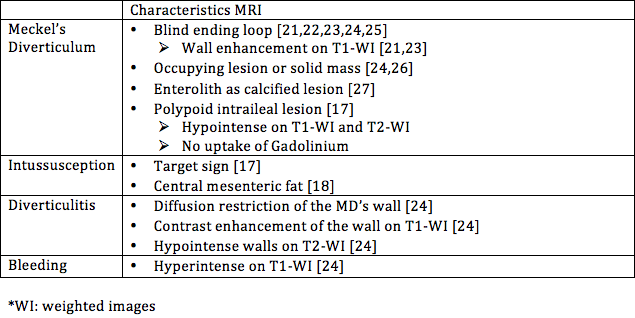
In the current available literature diagnosis of MD with MRI is only mentioned in case reports. Because of the lack of large studies, there are no reports on accuracy, sensitivity or specificity. One of the main advantages of MRI when compared to CT is the lack of radiation, which adds value especially for paediatric patients. Dujardin et al. described a polypoid intraileal lesion that had no contrast captation, which differs from our findings of contrast hypercaptation [17]. Intussusception can show as a Target-sign or as central mesenteric fat, the latter was present in our case as well [17,18]. Despite the potential of MRI, false negatives have been reported [19,20]. There is a need for large comparative studies to show whether the usage of MRI can be clinically relevant compared to CT, as well as to establish a reliable source for the radiological characteristics of MD. In Table 1 we summarized specific radiological characteristics for MD and its complications as described in the current available literature.
Despite the large array of imaging modalities, explorative laparoscopy remains the best diagnostic tool when Meckel’s diverticulum is suspected [1,2,3].
Surgical Treatment
In the current literature debate still remains whether preventive resection of incidental MD is indicated. Advocates claim that the risk for development of symptoms and malignancy justifies the need for resection of all incidentally found MD’s, whereas opponents claim that the risk of surgical complications outweighs the benefits of preventive resection [1,2]. The lifetime incidence of symptomatic MD is estimated between 4,2% and 9% [1]. Incidence of malignancy in MD has been reported between 0,5-5,1% [2]. In comparison, the lifetime risk for developing appendicitis is 7% and incidence of malignancy in resected appendices is 0,7-1,7% [2,28]. Of importance, there is a broad consensus to not resect the appendix prophylactically. An alternative solution is assessing the need for surgery based on the presence of risk factors for development of symptoms. In the literature review by Lindeman et al. it was proposed that presence of one of the following criteria indicated prophylactic surgery 1) <50 years, 2) male, 3) mesofibrous band and 4) length ≥ 2 cm [2].
Naturally, there is broad consensus pleading for surgical treatment when a Meckel’s Diverticulum becomes symptomatic. Studies have shown that laparoscopy is a safe and efficient technique for treatment of MD in comparison with laparotomy [29]. However, concerning the exact surgical treatment tree options exists: 1) diverticulectomy (tangential stapling or endoloops), 2) partial enterectomy and 3) wedge resection.
In the literature review by Blouhos et al. the surgical technique for MD-resection was based on two main principles. First, because ectopic tissue is associated with development of symptoms, surgery should always aim to resect all ectopic tissue [29,30]. Despite the lack of evidence for the hypothesis that leaving residual ectopic tissue in situ could cause recurrence of symptoms, it is a widely accepted recommendation in the available literature. Second, any bowel tissue that is affected by ischemia or inflammation should be resected as well. If the base of the diverticulum is affected by one of the above a formal enterectomy of the entire affected bowel segment should be performed [29].
The morbidity associated with simple diverticulectomy seems to be lower than that for enterectomy [31,32]. Although above findings are based on retrospective studies, they do suggest diverticulectomy being a safe and efficient surgical option. The downside of diverticulectomy is the risk of leaving ectopic tissue in situ, which could potentially be located at the base of the MD. Peroperative palpation and macroscopic histological aspect are deemed unreliable in determining whether ectopic tissue is still present or not [30]. If present, ectopic tissue can be located anywhere in the diverticulum ranging from the base to the top. However, a predisposition was found based on the height-diameter-ratio (HDR) [29]: In short and broad MD’s the ectopic tissue was spread diffuse including at the base of the MD, whereas long and slender MD’s have ectopic tissue in the tip or the middle. Cut-off values of 1,6-2 HDR were proposed for diverticulectomy above and enterectomy under. Peroperative frozen section can assure the presence of an ectopic tissue-free resection margin [33].
In the past it was stated that in the case of haemorrhaging MD, enterectomy was the only option, due to the continuing bleeding of residual ulcerated tissue in the adjacent ileum if diverticulectomy would have been performed [3]. On the other hand, recent findings showed that diverticulectomy is safe and efficient for haemorrhaging MD’s, concluding that residual ulcerated tissue will heal when all acid producing ectopic gastric tissue is removed [32].
Concerning the surgical management of intussusception of a MD there is still debate whether or not peroperative reduction is safe. The advantages of reduction are the possibility of simple diverticulectomy and smaller segmental resection in case of enterectomy. The possible disadvantages accompanied with reduction are: faecal soiling in case of perforation, potential seeding of malignant cells and a higher risk of anastomotic complications due to manipulated friable and oedematous bowel tissue [34,35]. To date there are no large studies focusing on this subject thus implying expert opinion as available evidence. Some authors state that reduction is a safe technique, because of the relative small chance of malignancy arising from MD’s [36]. This is in line with the recommendations for enteric intussusception in general, which has been studied more extensively. In a systematic review with meta-analysis it was concluded that enteric intussusception in contrast to colonic intussusception can be reduced, because the main malignancies causing enteric intussusception are metastatic carcinoma, lymphoma and GIST [37]. However, a literature review showed that the main malignant pathology arising in Meckel’s diverticula are carcinoid tumours (84,6%) [38]. It is important to realize that the surgical treatment differs when a carcinoid is present. The European Neuroendocrine Tumour Society recommended in their Guideline “en-bloc” resection with lymphadenectomy for gastro-intestinal carcinoid tumours irrespective of the size of the lesion [39]. In addition, assessing with CT whether underlying malignancy is present in the MD is known to be difficult [40]. Again, peroperative frozen section could aid in the decision process; when a carcinoid is expected more extensive resection with lymphadenectomy can be performed. In conclusion the safety of reduction of intussuscepted MD remains unclear, and further research is needed. However, we suggest diverticulectomy, even after reduction if the manoeuvre was easy and uneventful. Due to the minimal invasive nature of laparoscopy, more extensive surgery can still be undertaken on indication in second time, without added morbidity or delay due to the previous intervention.
Conclusion
Meckel’s diverticulum has a wide range of clinical features. This case report shows a rare insidious course with a total duration of 21 years. To date, preoperative diagnosis based on imaging alone remains difficult. There is no technical investigation with high accuracy, especially not in the adult population. MRI has potential to aid in the preoperative diagnosis and should be performed when CT cannot clarify the underlying pathology (as in this case). Laparoscopy is the standard for surgical treatment and the height-diameter ratio can aid in the decision process between diverticulectomy and formal partial enterectomy. Peroperative frozen section is advised when there is doubt about the resection margin as well as the presence of malignancy. Whether or not it is safe to reduce an invaginated MD remains subject of debate and can be left to the experience of the surgeon based on peroperative findings.
Conflict of interest: We have no conflicts of interest to disclose.