International Journal of Epidemiology And Public Health Research
OPEN ACCESS | Volume 9 - Issue 1 - 2026
ISSN No: 2836-2810 | Journal DOI: 10.61148/2836-2810/IJEPHR
Mbogo N. Kija 1,2, Sharadhuli S. Kimera 1, Ladslaus L. Mnyone 2
1,2 Msc. Epidemiology, Department of Public Health, Sokoine University of Agriculture, P.O. Box 3015, Morogoro - Tanzania
1 Professor of Veterinary Epidemiology & Infectious Diseases Sokoine University of Agriculture, College of Veterinary Medicine and Biomedical Sciences P.O. Box 3021, Morogoro - Tanzania
2 Professor of Entomology, Institute of Pest Management Sokoine University of Agriculture, P.O. Box 3110, Morogoro - Tanzania
1College of Veterinary Medicine and Biomedical Sciences, Sokoine University of Agriculture, P.O. Box 3015, Morogoro, Tanzania.
2Institute of Pest Management, Sokoine University of Agriculture, P.O. Box 3110, Morogoro, Tanzania.
*Corresponding author: Mbogo N. Epidemiology, Department of Public Health, Sokoine University of Agriculture, P.O. Box 3015, Morogoro - Tanzania.
Received Date: April 11, 2022
Accepted Date: April 21, 2022
Published Date: May 06, 2022
Citation: Mbogo N. Kija, Sharadhuli S. Kimera, Ladslaus L. Mnyone. “Retrospective Analysis of Malaria Cases in Selected Higher Education Institutions in Morogoro Municipality, Eastern Tanzania”. International J of Epidemiology and Public Health Research, 2(2). DOI:http;//doi.org/03.2022/1.1029.
Copyright: © 2022 Mbogo N. Kija. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background: The malaria transmission is largely dictated by climatic, topographic and ecological factors that vary widely from one area or cluster to the other, and to some extent we are lacking some basic background information on malaria especially in high learning institutions within Tanzania. Therefore, a need for more refined area specific interventions that would better consider the past record of malaria data for scrutinizing the disease trend so as to be in a position to act accordingly towards malaria elimination.
Methods: A retrospective study was conducted to determine a ten-year (2011 – 2020) trend on malaria prevalence based on patients who were attended from a catchment health facility in each institution. We used only malaria cases data diagnosed using either microscopy and/or malaria rapid diagnostic test (mRDT).
Results: A binary logistic regression test was significant for the test variables at p < 0.05 and the results showed that; males were likely to be malaria positive by 3.3 percent less compared to females, dry season by 2.6 percent less compared to wet season, Jordan by 2.3 times more than SUA and Mzumbe by 17.8% less as compared to SUA. Overall, SUA had many positive case (n= 27 320) followed by Mzumbe 23 690 and Jordan 14 959.
Conclusion: The overall trend of malaria positive cases seemed to decrease starting from 2017 onwards thou the observed prevalence (34.1%) is far high when compared to the national prevalence of 9.5% reported in 2017. Thus, we would recommend for the institutions to have a clear medical record system regarding student’s health status.
Malaria remains one of the major global public health problems accounting for about 435 000 deaths annually, 93% of the deaths occur in Africa 24. In Tanzania, the disease accounts for over 30% of the national disease burden, making it a top health priority for allocation of resources for its prevention and control 16 . Over the last decade, malaria incidence and mortality have significantly declined 23 .This is due to improved coverage of frontline mosquito vector control interventions, long lasting insecticidal nets (LLINs) and indoor residual spraying (IRS), and improved diagnosis and treatment 22,19 .As malaria transmission continues to decline, prevention and control strategies will increasingly need to rely on accurate knowledge of the spatial distribution of high-risk geographic areas to support elimination. The malaria transmission risk is largely shaped by climatic, topographic and ecological factors 18,10 as well as human behaviours and knowledge levels on the disease which vary widely from one person and or area to another 20,3 .This spatial heterogeneity in the transmission has resulted in malaria occurring in transmission clusters of varied environmental risk factors 7,8 both at the macro (e.g., temperature, precipitation) and the micro (e.g., local elevation, land use) spatial scales 6, 13 .Similarly, human socio-demographic characteristics such as sex, age, education level and residence of a person are important factors related to the disease transmission 5 .Seasonal variations are equally important factors influencing mosquito reproduction hence disease transmission 12, 2 . Furthermore, data availability on malaria for the past years is a challenge to many developing countries 11. This is due to the fact that as the World shifts to digital form of documentation, the analogy records are not backed up into an electronic format, as for the case in Tanzania 25, 4.
Despite of several sensitization campaigns and different interventional strategies made by the government and other stakeholders towards malaria elimination, still the outcome is unacceptably low among people in various clusters 14, 9. High learning institutions in Eastern Tanzania namely Jordan, Muslim, Mzumbe and SUA are better representatives of such clusters with high risk for malaria transmission. Therefore, if we are to win for the fight against malaria, such clusters would need more refined area specific interventions that would better suit this population. The intervention should consider factors like age, sex, education level, peer groups, exposure time and seasonality by tracking such indices through a retrospective analysis that would give a better trend of the disease and be in a position to act accordingly.
Methods
Study area
The retrospective malaria cases data were obtained from catchment health facilities of Sokoine University of Agriculture (SUA) (6.8278ºS, 37.6591ºE), Mzumbe University (MU) (6.9239ºS, 37.5691ºE) and Jordan University College (JUCO) (6.8068ºS, 37.7024ºE). Morogoro Municipality is about 200 km west of Dar es Salaam and lies between latitudes 5º7' and 10º00' South of the Equator and longitudes 35°6' and 39°5' East of Greenwich. Morogoro municipality experiences two main seasons: wet and dry season. The wet seasons run from March – May and October –December, with April and December being the wettest months. The dry seasons run from June – September and January – February, with July being the driest month. The area experiences an average rainfall of around 935 mm and temperature of around 24.6°C per annum. Within SUA campus there are some agricultural activities such as cattle keeping and growing varieties of crops year round as part of training. In Mzumbe only small part of the campus land is used for agricultural activities for growing vegetables and some seasonal crops like maize, rice, sweet potatoes, cassava and nuts. Muslim has only a very small portion of the delta that is mainly used for growing rice and some vegetables beside it. However, within Jordan campus there are no any agricultural activities carried out. On the periphery of these four institutions, people are largely involved in agricultural activities and they mainly grow maize, rice and vegetables. The landscape of these institutions is surrounded by several mosquito breeding sites of varied sizes which are temporal, semi-permanent and permanent.
Study design and population
A retrospective study was conducted to determine a ten-year (2011 – 2020) trend on malaria prevalence based on patients reports and test result registers.
Collection of malaria cases data
A ten-year (2011 – 2020) data on malaria cases was obtained from a catchment health facility in each institution. We used only malaria cases data diagnosed by using either microscopy and/or malaria rapid diagnostic test (mRDT). The required sets of information were extracted from patients’ register books and reports which included the reporting date/month, sex, age and lab results. The data were checked for completeness with close assistance from the laboratory personnel in the respective health facilities. However, institution “W” had no a nearby or common health facility where students get attended when sick, thus it was excluded and only three institutions (X, Y and Z) qualified for the study. Personal information of individual patients was excluded from the final dataset so as to observe confidentiality.
Statistical analysis
The data with appropriate coding was entered and cleaned in Ms Excel and finally transferred to SPSS version 22 IBM and Ms Excel computer programs for analysis, descriptive statistics are presented in tables and graphs. A p-value <0.05 was considered statistically significant for the test results in a binary logistic regression.
Results
A binary logistic regression was used to test for the significance of the variables with respect to the recorded Malaria cases data for ten years in each Institution. Three Institutions namely SUA, Jordan and Mzumbe had the records hence qualified for the test. They were five variables; malaria test result (positive or negative) serves as a dependent variable, age of the patient (Below 5 years and 5 years and above), sex of the patient (Male or Female), place/location where the patient tested (SUA, Jordan and Mzumbe) and seasonality (Dry or Wet) served as independent variables. All variables were statistically significant at 95% confidence Level. The results are summarized in Table 4.1.

Table 4.1: Binary Logistic Regression Output for Objective number 4
The probability of males having malaria positive result was 3.3 percent less compared to females, in patients aged below 5 years malaria cases were 11.2% less compared to patients aged 5 years and above, and dry season had malaria cases 2.6 percent less compared to wet season. With respect to place/location; Jordan was more likely to have positive malaria cases by 2-folds compared to SUA. Likewise Mzumbe is likely to have positive malaria cases by ~18% less compared to SUA (Table 4.1).
The proportion of positive malaria cases per institution: SUA had 27320 (33.0%) out of 82698, Mzumbe 23690 (28.8%) out of 82350 and Jordan 14959 (53.1%) out of 28195. Overall, the total positive cases when combined were 65969 (34.1%) and negative cases 127274 (65.9%) out of 193243. Total female malaria positive cases were 33415 (50.7%) out of 65969 and males 32554 (49.3%) out of 65969, in under-fives were 6290 (9.5%) out of 65969, in five and above years old were 59679 (90.5%) out of 65969 (Table 4.2) and for wet season 34493(52.3%) out of 65969 and Dry season 31476 (47.7%) out of 65969 cases (Appendix 4.1).

Table 4.2: Age category and malaria test result
The trend of malaria for the past 10 years among the three Institutions
The trend of malaria kept on fluctuating from year to year for which Mzumbe had a high number of malaria positive cases (Y, +ves) from 2011 to 2016 and it dropped drastically from 2017 to 2020 though there was a small rise in 2019. Jordan recorded a high number of Malaria positive cases (X, +ves) in 2014 and it sharply dropped from 2015 onwards and SUA had a steady like high number of positive malaria cases (Z, +ves) from 2011 to 2017 then it started going down from 2018 onwards (Figure 4.2). For individual Institutions see Appendix 4, 5 and 6.

Figure 4.2: A chart of positive malaria cases trend for the three institutions from the year 2011 to 2020
Key for Figure 4.2:
X, +Ves = Positive Malaria cases from Jordan Health facility,
Y, +Ves = Positive Malaria cases from Mzumbe Health facility
Z, +Ves = Positive Malaria cases from SUA Health facility.
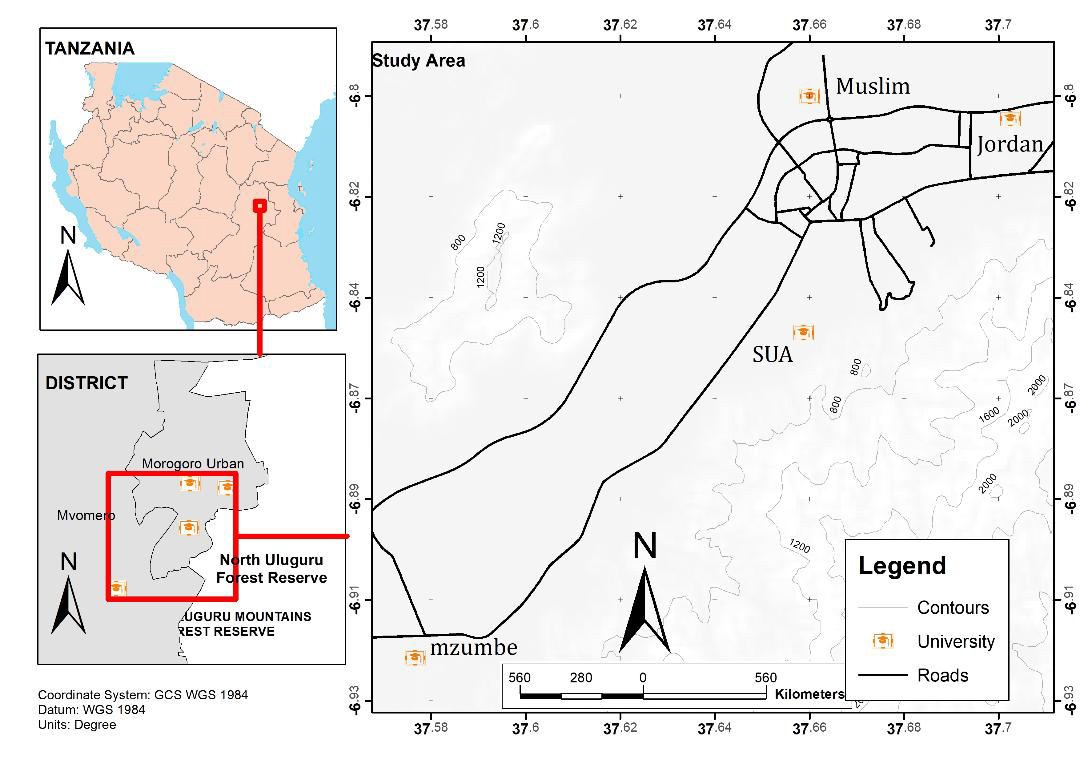
Figure 1: A map of Morogoro region showing the study higher education institutions (HEIs)
Malaria trend for individual Institutions
For Jordan University, malaria positive cases were leading compared to negatives from year 2011 to 2018 for which in the year 2014 there was a peak of about twice the previous years and in year 2017 positive malaria cases had a tinny drop as compared to negative cases. In year 2019 and 2020 negative cases were higher more than twice positive cases (Figure 4.3).

Figure 4.3: A chart of positive and negative malaria cases trend for Jordan University from the year 2011 to 2020
Meanwhile, the total number of malaria related patients decreased significantly from the year 2015 to 2020.
For Mzumbe University, negative malaria cases were higher throughout the 10 year period as compared to positive cases (Figure 4.4).

Figure 4.4: A chart of positive and negative malaria cases trend for Mzumbe University from the year 2011 to 2020
From 2011 to 2016 malaria positive cases were high and kept on fluctuating between 2 500 to 4 500 positive cases per year and there was an equal number of cases in 2016. However, 2017 and 2018 had the lowest number of positive cases with a slight increase in 2019 and 2020. Generally, the number of positive cases kept on decreasing as compared to negative cases from the year 2011 to 2020.
For SUA, the number of positive malaria cases seemed to be stead- like from year 2011 to 2019 with the number of cases ranging from 1 900 to 2 000 per year and year 2020 recorded the lowest number of less than 500 cases (Figure 4.5).

Figure 4.5: A chart of positive and negative malaria cases trend for SUA from the year 2011 to 2020
The negative cases were high throughout the decade and had a sharp increase in 2019 to 2020.
Information gained through management interview
Introduction and use of mRDT has contributed much to the variation in the number of positive malaria cases. The use of mRDT was mandated by the National guideline in malaria management that it should be used as the first line diagnostic tool as compared to microscopy. The intensive use of rapid test in Jordan and Mzumbe Universities started from the year 2017 and 2019 in SUA. The initiation of mRDT contributed to the difference and rise of negative cases as the tool seemed to have high specificity (as seen in the figures).
The number of patients was decreasing for Jordan and Mzumbe whereby at Jordan decreased significantly from about 5 000 to 1 800 patients per year. This was said to be attributed by the permission given to students that can be attended by other health facilities of their choice outside the University. Unlike for SUA as it was observed that the number of patients seemed to increase, this was said to be accelerated by the increase in students’ enrollment.
On the other hand, documentation of medical data was reported to be the most tiresome job because the same persons undertaking the tests (Laboratory technicians) are to document the data. Also, most of the data for the past years before 2018 are not transformed to electronic format rather are still in hard copies (books, registers and reports). Documentation problem was thought to be the result of qualified staff shortage in medical records.
Discussion
The test results showed a statistical significant difference among the variables of interest (malaria test result, Age group, Sex, place/location and Seasonality).
Socio-demographic variables particularly age and sex
Appendix 4.1 demonstrated that females were more affected by malaria by 3.3 percent more as compared to males (total female malaria positive cases were 33 415 (50.7%) out of 65 969 and males 32 554 (49.3%) out of 65 969) and many positive malaria cases were observed in patients aged 5 and above 5 years by 11.2% more than patients aged below 5 years (in under-fives were 6 290 (9.5%) out of 65 969, in five and above years old were 59 679 (90.5%). This concurs with the findings of the study on malaria transmission trends in Botswana 5. In line with this, Aikambe and Mnyone in their study at Mkuyuni and Kiroka of Morogoro region revealed the same except for the age group 1. The difference in age group is due to the fact that malaria cases data were from the health facilities within the Universities for which the majority of patients were students.
Seasonal variability with respect to malaria cases
With respect to seasons, wet season had many positive malaria cases by 2.6 percent more as compared to dry season (wet season 34 493(52.3%) and Dry season 31 476 (47.7%) as in Appendix 4.2. This corresponds with the retrospective study in Mpwapwa District of Morogoro for which malaria positive cases had a positive correlation with rainfall 15 and also in a study done in Kagera region 12. With respect to location; SUA had many positive malaria cases by 41.4% (27 320) followed by Mzumbe 35.9% (23 690) and Jordan 22.7% (14 959). The variation in malaria transmission is related to the varied environmental risk factors such as vegetation cover, land use, temperature, precipitation and local elevation 3, 13.
The trend of malaria for the past 10 years among the three Institutions
Generally, the overall trend of malaria positive cases has shown to decrease from time to time starting from 2017 onwards as revealed from all three Institutions. This could be due to the introduction and wide use of rapid malaria diagnostic test (mRDT) together with the positive outcomes of several interventions made by the Government and other stake holders. This is similar to the report by the United States Agency for International Development 21.
However, this study revealed a malaria prevalence rate of 34.1% that relates with the recent study in Morogoro, Tanzania by Aikambe and Mnyone 1 that observed a prevalence rate of 60.98%. This prevalence is higher than the 9.5% reported in 2017 by the Tanzania Ministry of Health 17 hence calling attention for further studies.
The results from this study observed that the overall trend of malaria positive cases kept on fluctuating from time to time but it has shown to decrease starting from 2017 onwards as revealed from the study Institutions. On the other hand malaria negatives have shown to increase from the year 2017 onwards. This could be due to the introduction and wide use of rapid malaria diagnostic test (mRDT) together with the positive outcomes of several interventions made by the Government and other stake holders. However, the observed prevalence (34.1%) is still unacceptably high and SUA had shown to have many positive cases with a steady like fluctuation followed by Mzumbe and Jordan.
Therefore, we would recommend that higher education institutions should have a clear medical record system regarding student’s illnesses and it has to be reviewed yearly so as to trace any changes for timely actions. Generally, the consumption of health data should be encouraged at the institutional level in order to have a clear understanding of student’s health status.
Also, the government should employ more staffs in health facilities especially on record keeping so as to have quality statistics for better planning at all levels.
List of abbreviations
CL Confidence Level
JUCO Jordan University College
mRDT Malaria Rapid Diagnostic Test
MU Mzumbe University
MUM Muslim University College of Morogoro
SUA Sokoine University of Agriculture
WHO World Health Organization
n Number
HIV Human Immunodeficiency Virus
AIDS Acquired Immunodeficiency Syndrome
P Level of significance
lab Laboratory
Ethics approval and consent to participate
Ethical approval for this study was obtained from the Research and Publication Committee of Sokoine University of Agriculture (SUA) having a reference no. SUA/DPRTC/R/06. Also a research permit was obtained from each institution and the surrounding community. Also the names of the institutions (Universities) were assigned identification letters namely W,X,Y and Z which in turn were coded as 1,2,3 and 4 respectively instead of their actual names in order to ensure confidentiality.
Availability of data and materials
The dataset used and/or analyzed, as well as the materials collected, during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no competing interests.
Funding
This study was conducted as part of the MSc research and was self-sponsored.
Authors’ contributions
MNK and LLM conceived and designed the study. GCK, MH, and SM collected the field samples. MNJ, SSK and LLM analyzed the data and coordinated the laboratory analyses. MNJ wrote the initial draft of the manuscript and LLM and SSK critically revised the manuscript. All authors have read and approved the final version of the manuscript.
Acknowledgments
We would like to express our deep gratitude to all who have in one way or another assisted for the accomplishment of this work through their assistance and professional advice. We also thank the senior management of all study HEIs for allowing us to conduct the study in their respective institutions and their cooperation throughout the study. The authors wish to especially thank the staff from the study HEIs, Health facility staffs, College of Veterinary Medicine and Biomedical Sciences and Institute of Pest Management (IPM) who in one way or the other contributed to this study.