International Journal of Epidemiology And Public Health Research
OPEN ACCESS | Volume 5 - Issue 1 - 2025
ISSN No: 2836-2810 | Journal DOI: 10.61148/2836-2810/IJEPHR
Akram Jassim Jawad
University of Babylon, College of Materials Engineering, Department of Polymer and Petrochemicals Industrials Engineering
Queen Mary University of London, School of Engineering and Materials Science, London, UK
*Corresponding authors: Akram Jassim Jawad, Queen Mary University of London, School of Engineering and Materials Science, London, UK.
Received: March 26, 2021
Accepted: April 02, 2021
Published: April 04, 2021
Citation: Akram J Jawad “Case Study; Ethical Issues in a Proposed Clinical Trial of a New Hip Prosthesis”. International Journal of Epidemiology And Public Health Research, 1(1); DOI: http;//doi.org/03.2021/1.1002.
Copyright: © 2021 Akram Jassim Jawad. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Surgery procedures of hip implanted have helped many people to enhance their function, mobility, and solve different problems such as osteoarthritis, fractures, birth defects, and some cases of post-traumatic states by inserting prosthetic implants of the femoral head and acetabulum. However, the random clinical trial has a significant role in the developing and proving process of products to be available on the market for the medical devices and treatments like hip prosthesis. This random procedure should be applied to simulate the natural differences in community such as sex, age, weight and ethnicity. Consequently, these procedures could rise to different ethical issues related to the physician investigators, participants and the community. In addition to that, these ethical issues play a significant factor in the acceptance and the approval process of successful medical products and devices. This work discussed the main ethical issues related with a proposed clinical trial of a new hip prosthesis, and compares it with an existing technique. These were achieved by applied four principles that are respect for autonomy, beneficence, non-maleficence, and justice, as well as the declaration of Helsinki.
Introduction
Implantation of hip prosthesis can help many people to enhance their function, mobility, and solve different problems such as osteoarthritis, fractures, birth defects, and some cases of post-traumatic states by using the surgery to insert prosthetic implants of the femoral head and acetabulum (Racine 2013; Coomber et al., 2016). There are about sixty thousand hip prostheses are implanted every year in the UK, with around sixty different types are used by the orthopaedic surgeon, which mostly need controlled clinical trials before they can be available on the market. Additionally, about 10-12% of all hip implants need revision, which introduce extra cost to the National Health Service (NHS), and decrease quality of patient life. Therefore, the number of new technologies of implantable devices in orthopaedic industries and medicine increases continuously, which are implanted by both younger and old traditional people, as well as professional people (Capozzi and Rhodes, 2015). Generally, there are two types of total hip prosthesis, which are cement type by using bone cement to attach the prosthesis and the bone, and cement-less by implanting directly into the bone by the osseointegration reaction (Colic and Sedmak, 2016). These procedures rise wide different and unique ethical issues that need to be balanced with technical needs to be acceptable by patients, their parents, society, physician and companies. Moreover, there is lack in the clinical trials studies of the comparison between the cement based and cement-less techniques of hip prosthesis, especially the medical ethics related. Where, most of these studies focus on the technical aspects like the implants position and the bone amount that be removed during the resurfacing process (Achten et al., 2010). Additionally, it is believed that the precise understanding of the main ethical issues such as generic concerns for all clinical random trials could play an important role in the valid consent process for participators in the trial.
In this report, the main ethical issues related with a proposed clinical trial of a new hip prosthesis have been described and discussed in details and compares it with an existing technique. In particular, the age of participants, and the high degree of invasive nature of implanting device were analysed with more attention. These were achieved by applied four principles that are respect for autonomy, beneficence, non-maleficence, and justice, as well as the declaration of Helsinki. Additionally, a proposal of information sheet and consent form were provided that could be used in the consent process of the clinical trial procedure of participants.
Clinical Trial
It is well known that there are different stages that need to pass it for any new proposal of medical devices, technology and treatments to be acceptable and to get approved as a product in the market, as we can see in the figure 1 (Racine, 2013). In the current case study, a clinical trial that is a controlled randomized study involves a comparison of the safety and efficiency between a new and an existed well-known hip prosthesis. Figure 2 shows schematic of the main parts of hip prosthesis as an implantable medical device (Racine 2013). The new hip prosthesis utilizes a new coating material that covers the stem, which has been designed and developed by JointSolve Ltd. In pre-clinical studies, this new coating material behaved as a biocompatible material in both in vitro and in vivo studies by using different animal models, which were Rabbit and Goat. This minimizes the existing problems of current prostheses, especially, aseptic loosening secondary to bone lysis.
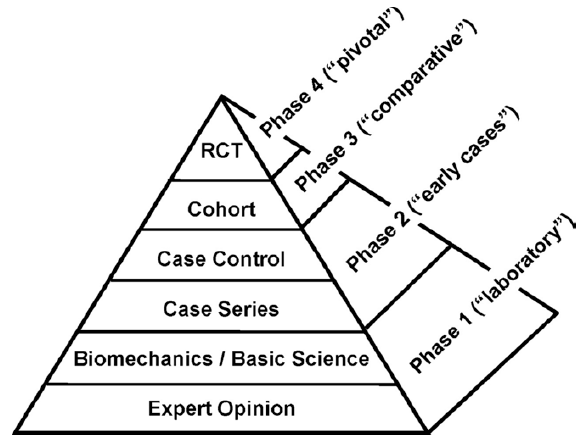
Figure 1: Different stages need to follow for new medical devices to get acceptance, and in product development, RCT= Randomized Controlled Trial (Racine, 2013).
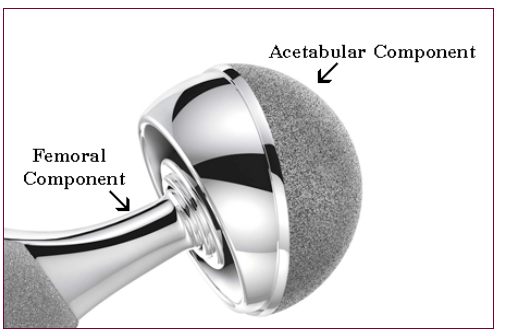
Figure 2: A schematic shows the main parts and types of hip prosthesis implantable (Racine, 2013).
Criteria and Procedure
The inclusion criteria of the current clinical trial study have included the current indications for standard primary total hip arthroplasty for non-inflammatory degenerative disease using cement-less components of acetabular and femoral. These have covered pain, deformity and loss of function, which are not responsive to medical treatment. However, criteria for specific exclusion have included inflammatory degenerative joint disease, previous joint infection, recent high dose corticosteroids or therapeutic radiation, metabolic disorders of calcifying tissue or people who are unable to attend for post-operative follow-up visits at the intervals detailed in the protocol.
The number of participants were two hundred from six different NHS centres in the UK. They were randomized to take either the new or the well-known prostheses with randomization stratified for aetiology and age, for a study period of ten years.
Follow-up appointments were six monthly in the first two years, while every year for the rest of years. The assessment have covered the clinical, pain, mobility and function, patient satisfaction and quality of life, clinical examination by surgeon and history of analgesia. In addition to that, it have covered radiographic (Anterior/Posterior and lateral of x-rays of the hip), as well as changes for movement in components of prostheses other than those that could be associated with patient specific factors. Additionally, evidence of bone lysis deemed secondary to the tracking of articular wear debris was covered.
Ethics Issues
In general, there are three main reasons for ethical issues in the current clinical trial, which are surgical procedures of hip implant, selection of material, and response of material. Some of these ethical issues could be specific to the current clinical trial that may not be working or found with other trials especially that are related with surgical procedures. To clearly that, different principles and criteria are considered to analysis the ethical issues of this clinical trial. For example, Tom Beauchamp and James Childress introduced four Principles for ethical issues in surgical procedures, which are respect for autonomy, beneficence, non-maleficence, and justice (Lawrence 2007). In addition to that, it is quite important to take in account these principles together along with the declaration of Helsinki 2000 to analyse the ethical issues more precisely (Carlson et al., 2004). However, in most cases these principles found come cross together in different levels and directions.
Regarding the age of the participants who are likely to be involved into the clinical trial, they have to be from different categories of ages, but they should be older than 18. Otherwise, there will be ethical issues about participation younger patients whom are less than 18 years, because of the decision-making ability. However, this age limit for decision-making ability could be changeable from country to another in the range 16-18 years, which needs to consider. That have been clarified clearly in article 10 from the declaration of Helsinki 2000 “Physicians must consider the ethical, legal and regulatory norms and standards for research involving human subjects in their own countries as well as applicable international norms and standards. No national or international ethical, legal or regulatory requirement should reduce or eliminate any of the protections for research subjects set forth in this Declaration" (Carlson et al., 2004). Although the younger candidates can participate due to taking parents’ permission, it will introduce ethical issues especially in the current clinical trial with high risks.
Regarding article 17 from the declaration of Helsinki 2000 "All medical research involving human subjects must be preceded by careful assessment of predictable risks and burdens to the individuals and groups involved in the research in comparison with foreseeable benefits to them and to other individuals or groups affected by the condition under investigation; measures to minimise the risks must be implemented; the risks must be continuously monitored, assessed and documented by the researcher" (Carlson et al., 2004). The risks and problems should be clarified clearly in the information sheet to inform the participants, which connect to autonomy and non-maleficence principles. For example, as this case study introduce a new coating material, there are a number of ethical issues could raise because of the natural meaning of the new treatment that is untested and unproven in any number of patients (Anand et al., 2011). Therefore, the potential complications and risks related should be clarified in the information sheet. That follow the main principles of medcial ethics that are firstly do no harm (nonmaleficence) and secondlly act for the good of our patients (beneficence) (Capozzi and Rhodes, 2015). Another example, the expected life duration of the existing hip prosthesis implants is between 10-15 years with four revisions. As an ethical need, the participants should know some important problems and limitations such as the amount of bone remaining after surgery, non-original form of regenerative bone if it is damage, the wear of the implant, and the unable nature of implant repair or grow (Gard et al., 2000). One-updated issues related with the infection that involve the high risk to be infected by Covid-19. It is necessary that patients be made aware of the risk of infection by viruses in this period of Covid-19 period. Therefore, patients can make their decisions regarding the current participation in the present Covid-19 risk (Kort et al., 2020). Therefore, the responsible on the current trial have an obligation to ensure that trial procedures are carefully designed and high clarified in the information sheet to minimize related different risks to health participants, and to inform them.
The main ethical principle of autonomy refers that the participant has full right to decide well-informed decisions based on an information sheet for their participation in clinical trials (Silber 1992). That mean it is important to clarify all necessary data in the information sheet, which may be an ethical problem as the new device have a limited and sometimes unsure data input by the investigators. In this case, the preclinical data need more investigations; with more analysis, to be sure the benefits outweigh the risks for the clinical participants. That agree together with article 16 from the declaration of Helsinki 2000 "In medical practice and in medical research, most interventions involve risks and burdens; medical research involving human subjects may only be conducted if the importance of the objective outweighs the risks and burdens to the research subjects" (Carlson et al., 2004).
As a beneficence principle, one of the main principles in medical ethics that is the physician’s obligation to act for the good of the patient (Capozzi and Rhodes, 2000). Which also agree with article 7 from the declaration of Helsinki 2000 "Medical research is subject to ethical standards that promote and ensure respect for all human subjects and protect their health and rights" (Carlson et al., 2004).
Another issue related with the learning curve which decide the surgeon if it is experienced or not, especially in the case of the more complex and new implanted hip prosthesis. For example, if the surgeon has lower experience, this might introduce high risk, discomfort, and potential complications to the patient (Capozzi and Rhodes, 2015). In other words, there are different factors related to the surgeon’s experience that could introduce risk, which include operating time, implant material choice, component positioning, and intraoperative difficulties (e.g., fracture, nerve and vascular damage) (Namba et al., 2013; Jameson et al., 2013). This could come cross on another important principle in medical ethics is that do no harm for the patient, which be against non-maleficence principle as well. Consequently, it is quite important to select the appropriate surgeon with appropriate experience, and clarify that in the information sheet for the patients to be informed (Capozzi and Rhodes, 2000; Edelstein et al., 2014).
Other factors not related to the surgeon’s experience, but they are related to participants that mainly appear after the implantation process. These include bacterial colonization, diabetes control, body mass index (BMI), smoking status, fall risk, narcotic and/or alcohol dependence, physical conditioning, neurocognitive disorders, nutritional status, cardiovascular status, non-genetic thromboembolic risk, and anaemia. However, some mentioned factors especially that related to participant are manageable, which introduce ethical issues, such as whether the participant mange and solve these risk factors, and to what extent before they participate in the clinical trial.
However, the essential reason behind participation people in even high risk trial because they understand that continuing developing in medical devices and treatments is important to solve disses and problems in health (Capozzi and Rhodes, 2000; Edelstein et al., 2014). Which means that they think this new coating material may be fill the gap between the humanity needs and the existing hip prosthesis.