Chrisostomos Sofoudis 1*, Andria Peraki 1, Angeliki Mavroidi2, Evangelia Platsouka2 and Efthimios Papamargaritis1
1 Department of Obstetrics and Gynecology, Konstandopoulio General Hospital, Athens, Greece.
2 Department of Microbiology, Konstandopoulio General Hospital, Athens, Greece.
*Corresponding Author: Chrisostomos Sofoudis, Department of Obstetrics and Gynecology, Konstandopoulio General Hospital, Athens, Greece.
Received date: January 11, 2021
Accepted date: February 18, 2021
Published date: February 24, 2021
Citation: Sofoudis C, Peraki A, Mavroidi A, Platsouka E and Papamargaritis E, Sphingomonas Paucimobilis Complicating Vaginal Fluid Cultivation. A Rare Case. International J of Clinical Gynaecology and Obstetrics, 2(1); DOI: http;//doi.org/03.2021/1.1009.
Copyright: © 2021 Chrisostomos Sofoudis. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Sphingomonas paucimobilis represents an aerobic Gram-negative bacillus that is gaining recognition as an important human pathogen.
These species are widely distributed in both natural environment and hospitals.
They appear as opportunistic pathogen that take advantage of underlying conditions and diseases. Regardless of the clinical significance, pathogenic mechanism varies throughout current bibliography.
Aim of our study, reflects presentation of a rare case of an out-patient clinical asymptomatic, with vaginal culture positive for this rare microorganism, S. paucimobilis.
Assiduous diagnosis and therapeutic mapping consist necessary conditions of effective treatment.
Introduction
Sphingomonas paucimobilis depicts a glucose-no fermenting, strictly aerobic, Gram-negative bacillus that thrives in the natural environment, especially in water and soil.
At the same time, it has also been isolated from hospital settings, including hospital water systems, distilled water, dialysis fluid, nebulizers, and other respiratory therapy equipment. [1-4]
S. paucimobilis has the property of being found in low carbon environment (oligotropic). It is used for bioremediation of the environment for its ability to decompose aromatic compounds. [5]
Additionally, is characterized by catalase and oxidase activity, yellow pigment production, and slow motility with single polar flagellum. [6]
S. paucimobilis was initially reported to cause human infection in 1979. It consists an opportunistic pathogen, isolated from blood, sputum, urine, wound, bile, cerebrospinal fluid, vagina, and cervix.
Its virulence is thought to be low, and identification of the organism from clinical specimen is rare.
However, it is associated with great variety of infections, concerning community-acquired and health care-associated ones.
Specifically, literature has documented bacteremia, pneumonia, catheter related infections, meningitis, peritonitis, osteomyelitis, septic arthritis, postoperative endophthalmitis, lung empyema, splenic abscesses, urinary tract infections, and biliary tract infections. [7-26]
Community-acquired infection, diabetes mellitus, and alcoholism have been established as significant risk factors for primary pseudomonada paucimobilis bacteremia. [27]
Case
We present a case of a 72- year old female patient (G4, P4), with history of hypertension and hypothyroidism pharmaceutically regulated, admitted at our Department with ultrasonography depiction of uterine cavity filled with fluid presence.
Pap smear revealed no signs of malignancy. Abdominal MRI depiction confirmed fluid presence inside uterine cavity along with intense heterogeneity of uterine configuration.
Patient underwent hysteroscopic evaluation of uterine cavity along with diagnostic curettage.
During cervical penetration, a massive amount of yellow-pigmented fluid was noticed.
A cotton swab filled with suspected fluid sent for microbiologic evaluation and cultivation.
The sample from pus was cultured at 35 0C for 24-hours and yielded yellow-pigmented, smoothed, convex, non- haemolytic, oxidase- and catalase-positive colonies, growing on blood agar (Figure I) and chocolate agar plates, but not on McConkey agar plate.
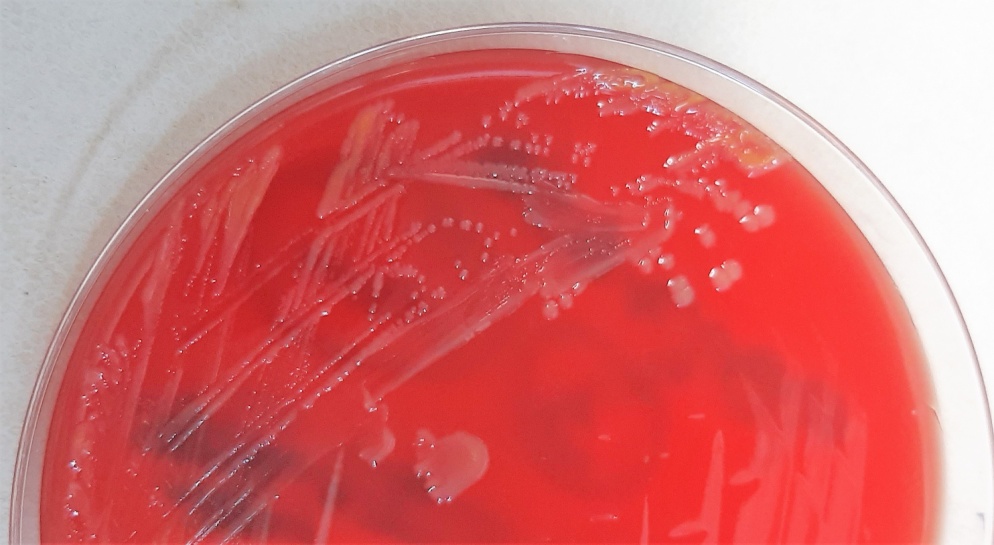
Figure I: S. paucimobilis culture on blood agar showing yellow-pigmented colonies.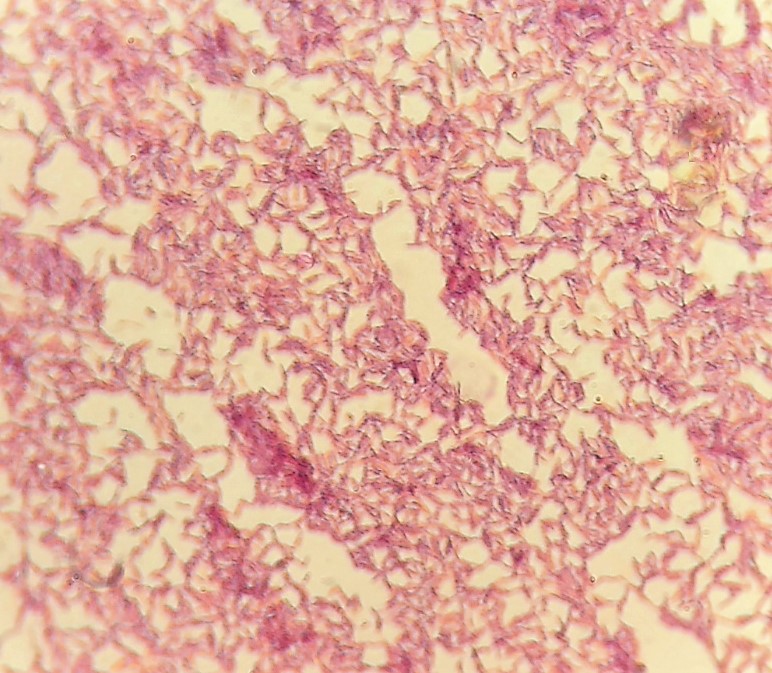
Gram stain showed gram-negative bacilli (Figure II), which were identified as Sphingomonas paucimobilis by the VITEK® 2 Compact15 automated system (Biomereux, France).
Figure II: Gram stain of the gram-negative bacillus S. paucimobilis.
Antibiotic susceptibility testing was performed both by the VITEK® 2 Compact15 automated system and the ETEST® method (Biomereux, France) on Mueller Hinton agar plates using an inoculum of 0.5 McFarland and a 24-hour incubation at 35 0C.
The isolate was characterised as: resistant to ceftazidime (MIC> 32μg/ml) and piperacillin/tazobactam (MIC> 16μg/ml, ciprofloxacin (MIC=1 μg/ml), susceptible-increased exposure to imipenem (MIC=2 μg/ml), and susceptible to meropenem (MIC=1 μg/ml) and amikacin (MIC<=2 μg/ml), according to the EUCAST 2020 criteria for Pseudomonas aeruginosa.
Hysteroscopic evaluation of uterine cavity did not reveal signs of uterine malignancy. Histologic examination of diagnostic curettage consisted areas of cervical and endometrial infection.
Patient discharged from hospital the folowwing day in good clinical condition. She reveived tb Amoxicillin and clavounic acid (Augmentin) for 10 days, 2 times per day.
Follow up with new vaginal fluid cultivation did not reveal presence of S. paucimobilis
Discussion
S.paucimobilis consists an aerobic, non-fermentative gram negative bacterium, widely distributed in the natural environment (e.g. water, soil).
It represents an opportunistic pathogen, considered to originate from contaminated hospital equipment or manipulation of some medical devices, causing hospital infections in immune-compromised patients and very rare in healthy persons.
Balkwill et al. reported that, although there may be certain predisposing conditions, it may not be always possible to determine the source of the infection. [28]
Lin et al. evaluated 16 cases with S. paucimobilis bacteremia and found a malignancy rate of 57.1% and a diabetes rate of 40.5%. [29]
So far, a variety of infections have been reported with this microorganism, such as sepsis, septic pulmonary embolism, septic arthritis, peritonitis, and endophthalmitis.
According to Cheong et al., the most common type of infection was catheter-related infection (34.8%) followed by primary bacteremia (26.1%), continuous ambulatory peritoneal dialysis peritonitis (13.0%), and gastrointestinal infection (8.7%). [30]
Disclosure of interest: All authors declare any financial interest with respect to this manuscript.
Conclusion
S. paucimobilis represnts a Gram negative bacillus, relative rare isolated in gynecologic speciments.
Assiduous therapeutic mapping consists ultimate scope, in order to avoid systemic infections and increase of patient’s quality of life.