Immunity Inflammation and Infection Diseases
OPEN ACCESS | Volume 2 - Issue 1 - 2025
ISSN No: - | Journal DOI: 10.61148/IIID
Sam Shan1*, Andrew Foote2, Mueed Mian2
1Department of General Medicine, Northern Health, Victoria, Australia.
2Department of Rheumatology, Northern Health, Victoria, Australia.
*Corresponding author: Sam Shan, Department of General Medicine, Northern Health, Victoria, Australia.
Received Date: February 15, 2024
Accepted Date: February 20, 2024
Published Date: February 29, 2024
Citation: Shan S, Foote A, Mian M. (2024) “Myositis Immunoblot - Who Orders them and What are the Results? A Retrospective Audit of Two Large Referral Centers.” Immunity, Inflammation and infection Disease, 1(1); DOI: 10.61148/IIID/001.
Copyright: © 2024 Sam Shan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
Idiopathic inflammatory myopathies are a group of disorders associated with myositis-specific antibodies (MSA) and myositis-associated antibodies (MAA) investigated through myositis immunoblot (MIB) (1). Due to the variable clinical presentations of these conditions, MIB is helpful to classify clinical syndromes with both treatment and prognostic implications (2). We report the ordering patterns of MIB and MAA in two large referral centers in Melbourne, Australia.
Methods
We identified all patients who underwent MIB testing between January 1 2019 and December 31 2020, there were no cut off periods for symptom duration or follow up period. No patients were excluded from this study. Relevant demographic, clinical data and additional investigations were obtained by chart review.
Results
Over the 2-year study period, 294 MIBs were ordered. Of these, 60 (20.4%) were positive. The median age was 62. The most frequent indication for MIB testing was onset of respiratory symptoms and the mean time of testing from onset of symptoms was 20 weeks (IQR 6.9-104). Respiratory (41%), rheumatology (24%) and neurology (15%) ordered the most MIBs. Rheumatology was consulted in 41% of the MSA ordering and also had the highest positive rate of the three specialties (rheumatology 30.5%, neurology 20.5%, respiratory 18.5%). Anti-SCL100 was the most common MSA present (16.7% (n = 10)). Positive MIB results were helpful in the final diagnosis of 20 participants (33.3%)
Conclusion
Among MIB studies performed, 20.4% of results were positive with most common being SCL100. Respiratory service ordered the largest number of tests predominantly to evaluate interstitial lung disease (ILD), a higher proportion of tests recommended by the rheumatology service was positive.
myositis, immunoblot, autoantibody, interstitial lung disease
Introduction
Idiopathic inflammatory myositis (IIM) is a spectrum of conditions involving multiple body systems. Serum autoimmune antibodies are frequently detected in conjunction with these conditions, classified as myositis specific antibodies (MSA) and myositis associated antibodies (MAA) (1). Autoantibodies play a crucial role in profiling clinical syndromes, providing prognostic insights, and assisting in therapeutic decision-making based on recognized syndromes (2). There is heterogeneous presentation of these conditions encompassing musculoskeletal, interstitial lung disease (ILD) and dermatological manifestations (3). MAA encompasses a wide array of investigations including anti-nuclear antibodies (ANA) and extractible nuclear antigen (ENA) and implies a reduced specificity for IIM than MSA (4). The patterns of MSA and MAA positivity contribute to subtyping the clinical syndrome, guiding further investigation, management, and prognostic considerations (5).
Anti-aminoacyl tRNA synthetase (ARS) antibodies are linked to antisynthetase syndrome, associated with improved survival but a higher recurrence rate(1, 6, 7). Conversely, anti-melanoma differentiation gene-5 (MDA5) positivity serves as a specific antibody for dermatomyositis and an independent risk factor for ILD (8, 9). The prevalence of anti-TIF1ɣ and anti-NXP2 is notably lower in IIM, with close associations with malignancies (10).
Testing for MSA is both costly and labor-intensive, typically conducted through immunoblot (IB), with alternative methods including immunoprecipitation (IP) or enzyme-linked immunosorbent assay (ELISA) (11). The prevalence of MSA is relatively low, with anti-Jo1 being the most commonly identified MSA, present in 30% of IIM patients (11). Importantly, MSA positivity is not a mandatory requirement for IIM diagnosis, and anti-Jo1 was the only MSA included within the 2017 EULAR/ ACR classification criteria for adult and juvenile idiopathic inflammatory myopathies (12). Therefore, ordering MSA and MAA testing should be approached judiciously and based on clinical suspicion.
Despite the widespread ordering of MSA in Australia, scant data exists regarding the clinical indications and their impact on clinical practice. The objective of our study is to delineate the pattern and outcomes of MIB (encompassing MSAs and MAA) ordering across two major Australian public hospitals, and to examine any subsequent investigations performed in these patients.
Methods
We retrospectively reviewed the clinical context of all MIB tests ordered in the two year period between January 1st 2019 and 31st December 2020 at Austin Health and Northern Health, two major health networks in Melbourne, Australia. No exclusion criteria applied to this study to provide insight into the ordering pattern of MIB rather to select for known diseases such as past literature. MIB is performed via the Euroline Autoimmune Inflammatory Myopathies 16 Ag (IgG) assay which encompasses antibodies against Mi-2a, Mi-2b, TIF1ɣ, MDA5, NXP2, SAE, Ku, PM-SCL100, PM-SCL75, Jo-1, SRP, PL-7, PL-12, EJ, OJ and Ro-52. The MIB panel is ordered together and its components could not be ordered individually. There were no exclusion criteria to give a comprehensive overview of the ordering pattern.
Basic patient demographic data was recorded. MIB was recorded as negative or the positive antibody, if multiple MIB panels were ordered and performed, all positive antibodies are recorded. MAA was recorded as either negative or positive with titre and pattern for ANA, while ENA was recorded as positive or negative with specific antibody for ENA. Speciality team ordering the test and clinical details surrounding ordering of MIB including type and duration of symptom onset and other positive laboratory or radiological findings were recorded, we also noted whether Rheumatology as a speciality was involved in their care prior to conducting MIB investigation. Notably, these documentations are not restricted by the 2 year time frame, inpatient vs outpatient setting or the number of visits prior to testing. For those who were MIB positive, we recorded all further investigations performed either for confirmation of diagnosis or complication screening, these include muscle or skin biopsies, magnetic resonance imaging (MRI), computed tomography (CT), positron emission tomography (PET), spirometry and electromyography (EMG). Creatine kinase (CK) at date of first presentation and final clinical diagnosis was also recorded.
Data was collected on Microsoft Excel 2020 and statistical analysis was performed on RStudio 1.3.1073 using package tidyverse. Local ethics committee approval was granted prior to commencement of the study (Audit 21/06).
Results
Patient demographics
Over the two year study period, a total of 294 MIB were ordered. The median age was 62 years (IQR 47 – 74), 148 (50.3%) of the participants were male. The majority of the patients (73.1%) were of Caucasian ethnicity, 7.1% were Middle Eastern and 5.8% were Asian, 11.5% of patients did not have ethnicity stated in their file. The remaining 2.4% of the participants were made up of Hispanic, Islanders and Sub-Saharan Africa ethnicity. The median time between symptoms onset and MSA test ordering is 20 weeks (IQR 7 – 104). The median CK reading was 111 units/ L (IQR 59 – 300).
Table 1: patient demographic data
|
Demographics |
n/ Median |
IQR |
|
Age |
62 |
47 – 74 |
|
Male sex (%) |
148 (50.3%) |
|
|
Total MIB ordered |
294 |
|
|
Rheumatology involvement (%) |
121 (41%) |
|
|
MIB positive (%) |
60 (20.4%) |
|
|
Median time from symptoms to MIB testing (weeks) |
20 weeks |
7 – 104 |
MSA ordering indications and patterns
Documentation indications triggering MIB testing was present in only 65% of cases. Respiratory symptoms including dyspnoea and chronic cough were the primary indications for MIB testing in 30.6% of the cases, constituting the most common indication. Incidental radiological changes suggestive of ILD was the second most common indication and accounted for 17% of the cases. 12.6% of the cases had musculoskeletal symptoms (including bulbar symptoms). Rashes suggesting a dermatomyositis-like illness was the indication in 9.2% of the participants. Arthritis and/or arthralgia were the presenting symptom/s in 7.8%, while dysphagia was the indication in 6.1%. MIB was performed solely due to elevated CK without musculoskeletal symptoms in 3.7% of the participants (Table 3).
Table 2: Speciality ordering MIB
|
Speciality ordering MIB |
N (% of total) |
|
NA |
14 (4.8%) |
|
Cardiology |
1 (0.3%) |
|
Dermatology |
6 (2.1%) |
|
Endocrinology |
1 (0.3%) |
|
Gastroenterology |
1 (0.3%) |
|
General medicine |
12 (4.1%) |
|
Neurology |
48 (16.3%) |
|
Oncology |
2 (0.7%) |
|
Radiology |
1 (0.3%) |
|
Renal |
2 (0.7%) |
|
Respiratory |
122 (41.5%) |
|
Rheumatology |
84 (28.6%) |
|
Total |
294 |
Table 3: Indications for MIB testing
|
Indication for testing |
N (% of total) |
|
NA |
89 (26.4%) |
|
Arthritis/ arthralgia |
23 (6.8%) |
|
Bulbar symptoms |
19 (5.6%) |
|
CK |
11 (3.3%) |
|
Constitutional symptoms |
7 (2.1%) |
|
Musculoskeletal |
23 (6.8%) |
|
Paraesthesia |
1 (0.3%) |
|
Ptosis |
1 (0.3%) |
|
Rash |
28 (8.3%) |
|
Respiratory |
130 (38.6%) |
|
Serositis |
3 (0.9%) |
|
Sicca |
2 (0.6%) |
|
Total |
337 |
NB: Counts are repeated in multiple indications.
Majority of the MIB testing were ordered by three specialities – rheumatology, respiratory and neurology. Respiratory was the most common ordering speciality, ordering 41% of the tests, rheumatology ordered 24% while neurology ordered 15% of total tests (Table 2). Other specialities ordering MIB included dermatology, cardiology, endocrinology, gastroenterology, renal, oncology and general medicine. Dermatology and nephrology also contributed to positive MSA testing however their numbers were small (3 and 1 positive test respectively). 41% of patients who had MSA testing also had reviews by the rheumatology department. We also compared the rate of positive MSA test between the three main specialities. Rheumatology had the highest rate of a positive test (22; 30.5%), followed by respiratory (25; 20.5%) and neurology (8; 18.2%). Rheumatology was also the most likely department to order MSA based on multiple clinical features compared to other specialities (17.8% vs 7.7%).
MIB results
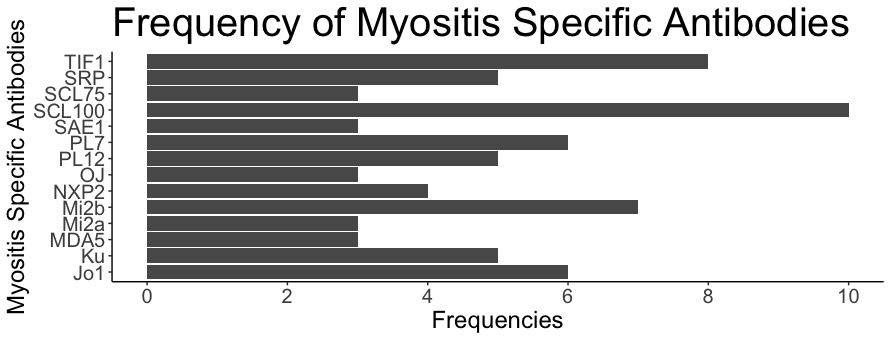
A positive MIB result was present in 60 (20.4%) participants. 51 participants tested positive for a single MSA, seven participants tested positive for two, and two tested positive for three MSA. The most common MIB present was SCL (10; 14.5%), followed by TIF1ɣ (8; 11.6%) and Mi-2b (7; 10.1%) (Graph 1). There was a total of 6 Jo-1 positive patients (10%). ANA was requested in 275 while ENA was requested in 266 of the 295 patients. ANA was found to be positive in 147, (53.4%), the most common titre was 1:160 (50; 34%) followed by 1:80 (36; 24.5%). The most common ANA pattern was speckled (107; 72.7%) followed by homogeneous (43, 29.3%) and 24 participants showed more than one ANA pattern. ENA was positive in 28 (10.5%) for a total of 39 antibodies, Ro was the most frequent ENA (8, 20.5%). Those who are MIB positive are significantly more likely to be ANA but not ENA positive (p <0.01).
Within the 60 cases of MIB positive group, 25 (42%) had muscular involvement, 32 (53%) had respiratory system involvement and 6 (10%) had involvement of both muscular and respiratory systems. Further diagnostic tests including MRI myositis protocol in 13 (22%), EMG in 5 cases (8%), muscle biopsy in 10 (15%) and skin biopsy in 5 (8%). 32 (53%) received a high resolution CT (HRCT) chest for further evaluation, eight (25%) of which had a normal HRCT. Malignancy screening was performed in eight of 14 participants (57%) who tested positive for NXP2, TIF1ɣ or SAE1 (malignancy associated MSA), in contrast, only six (13%) of participants who are positive for non-malignancy MSA underwent malignancy screening. No malignancy was confirmed in our study.
ILD was the most common extramuscular manifestation. Of the 32 patients receiving HRCT for further evaluation of their disease, 26 (82.2%) returned a positive result suggestive of ILD. Ground-glass changes were present in 12 (37.5%), reticulation was present in 5 (15.5%), non-specific fibrosis was present 5 (15.5%) and honeycombing was present in 2 (6.3%). The location of the HRCT changes was basal predominant 17 (40.6%) and tend to be bilateral (23; 71.8%). A radiological pattern was seen in five participants, usual interstitial pneumonia (UIP) pattern was observed in four participants while non-specific interstitial pneumonia (NSIP) pattern was observed in one participant.
During the retrospective study period, 39 (65%) of the MSA positive participants were given a final diagnosis. Idiopathic ILD was the final diagnosis in 14, 13 were diagnosed with dermatomyositis (DM), six with polymyositis (PM) and six had mixed connective tissue disease (MCTD). Of this group, 10 (16.7%) had ILD relating to connective tissue disease (7 DM, 2 PM, 1 MCTD). Only 1 participant satisfied the Bohan and Peter Classification Criteria for definite PM or DM while 3 participants satisfied the criteria probable PM or DM. 5 participants satisfied the ACR/ EULAR Classification Criteria 2017 for PM or DM. 28 (35%) of MSA positive patients are yet to be given a conclusive diagnosis. Positive MIB results were beneficial in the final diagnosis of 20 participants based on the review (33.3%).
Figure 1: Bar graph of frequencies of antibodies from Euroline Autoimmune Inflammatory Myopathies 16 Ag (IgG) assay, only positive data is displayed.
Discussion
The cumulative MIB positivity observed across a 24 months span within two major Australian health services was 20.4% and the most frequent MIB test result was SCL100. In contrast to previous investigations citing anti-Jo1 as the most prevalent MIB constituting between 15-25% of the cohort, our study exhibited a distinct outcome with anti-Jo1 frequency of 8.7% (11). Noteworthy is the fact that, despite our participant demographic primarily comprising individuals of European descent, our anti-Jo1 prevalence was significantly lower than that reported in extensive European cohorts (13). A marked disparity was identified in our study, revealing elevated rates of participants testing positive for more than one MSA within our cohort, a phenomenon not mirrored in a preceding European-based study (15% vs. 0.2%). Intriguingly, Anti-SCL100 emerged as the most frequently detected MIB in our study, a known association with systemic sclerosis (SSc), despite the absence of SSc diagnoses (14).
An in-depth analysis of MIB test ordering practices across the two major public health services disclosed that 80% of these tests emanated from the collaborative efforts of respiratory, rheumatology, and neurology departments. Notably, although the respiratory department accounted for the majority of test orders, the rheumatology department exhibited the highest rate of positive test outcomes. This nuanced dynamic is presumably rooted in divergent pre-test probabilities and varying levels of experience in managing MSA-related diseases. Traditionally, rheumatologists oversee the management of idiopathic inflammatory myopathies (IIM), potentially explaining their propensity to prioritise clinical findings over laboratory testing and utilise MIB for confirming clinical diagnoses. Our findings in parallel those of Maheswaranathan et al’s study (15) where 26.4% of the MIBs lacked recorded indications, attributed to a combination of inadequate documentation and external pathology requests.
The intricacies of MIB positivity reporting are compounded by differences in assays and the classification of weak positives across laboratories. Commercial MIB assays exhibit disparate performance for distinct MSAs and MAAs, with variations across laboratories (16). Notably, strongly positive results are more suggestive of IIM, ILD and connective tissue diseases in contrast to weakly positive MIB (17). The literature, as reviewed by To et al. underscores the likelihood of false positives in weakly positive MSA cases in up to 28.6%, while weakly positive MAAs tend to be more indicative of true positives with only 4% considered to be false positives (18). Within our cohort, where nine participants tested positive for multiple MIB antibodies, a suspicion arises regarding potential false positives, considering the conventional mutual exclusivity observed with MSAs (19).
Divergent international perspectives on MIB testing in the context of ILD further complicate the landscape. The Thoracic Society of Australia and New Zealand advocates for the integral role of MSA in ILD diagnosis, echoing the recommendation for rheumatological or immunological input in result interpretation (20). This aligns with the American Thoracic Society's Clinical Practice Guide, where routine rheumatological panels, including MSA, are recommended for all new ILD diagnoses (21). Conversely, the British Thoracic Society dissuades serological testing for ILD (22). Our study mirrors the practices of respiratory specialists in favour of the guidelines set by the Thoracic Societies of America, Australia, and New Zealand. Additionally, it sheds light on the overspecific nature of existing IIM classification criteria, with only a minority of clinically diagnosed polymyositis (PM) or dermatomyositis (DM) participants fitting the Bohan and Peter Classification Criteria or the ACR/EULAR Classification Criteria 2017.
In the absence of specific protocols for post-positive MSA/MIB findings, further investigations were warranted in certain scenarios to confirm diagnoses or exclude complications. Confirmatory measures included MRI of muscles, muscle biopsy, and/or skin biopsy for PM or DM, especially when clinical findings were insufficient for diagnosis. High-resolution computed tomography (HRCT) was employed to assess the possibility of ILD, and malignancy screening via positron emission tomography (PET) or CT chest abdomen pelvis was more likely for individuals with positive MSAs associated with malignancy.
To the author’s knowledge, this is the only study in Australia to investigate the pattern of MIB testing between specialities, with a comparable study by Maheswaranathan et al based in United States (15). Both studies share a predominant focus on Caucasian participants and exhibit analogous MSA testing patterns. Interestingly, the observation that Jo-1, often cited as the most frequent MSA in cohorts with identified rheumatological diseases did not emerge as the most frequent MSA in our study, aligning with findings of other audits of MSA (11, 15). Distinct from the American cohort, our study presented a more diverse panel of MSAs, breaking down groups of autoantibodies into individual counterparts such as Mi2a, Mi2b, SCL75, and SCL100. While this expanded panel theoretically allows for a more nuanced characterisation of clinical syndromes, statistical significance could not be established due to the limitations posed by our modest sample size. We suspect the differences in health care models between Australia and United States may also affect the generalisability of the studies.
Limitations inherent to this study encompass its retrospective design and the relatively small sample size. The retrospective chart review revealed instances of missing data attributed to poor documentation, introducing a potential source of bias. The study design is susceptible to recall and misclassification bias, as data collection relied on clinician-documented information, often lacking specificity regarding the rationale for MSA testing or the performance of other tests, shaped by personal experiences with the respective conditions.
In conclusion, our study evaluated the pattern of MIB ordering across two major public health services encompassing 294 MSA panels over a two year period. Noteworthy findings include the respiratory department accounting for the majority of test orders (41%), while the rheumatology department exhibited the highest rate of MIB positivity (30.5%). Respiratory symptoms emerged as the most common non-musculoskeletal indication for MIB testing, with SCL100 being the most prevalent MIB. Despite mirroring international trends in testing patterns, our study unveiled a diverse MSA profile. Given the integral role of MIB testing in ILD diagnosis we recommend interdepartmental education in clinical context indicating MIB testing and advocation for early rheumatological review prior to MIB testing to improve health economics and collaborative learning. Prospective cohort studies of MIB-positive patients are warranted to explore the timing and mode of follow-up investigations, as well as potential changes to diagnoses over time.
Acknowledgements: nil
Key points