Parveen Malhotra*, Vani Malhotra, Usha Gupta, Paramjeet Singh Gill, Pushkar, Yogesh Sanwariya
Department of Medical Gastroenterology and Microbiology, Gynecology & Obstetrics, PGIMS, Rohtak & Director Health Services, Haryana, India
*Correspondence author: Parveen Malhotra,128/19, Civil Hospital Road, Rohtak, Haryana, India (124001).
*Correspondence author: Parveen Malhotra,128/19, Civil Hospital Road, Rohtak, Haryana, India (124001).
Received date: February 03, 2021
Accepted date: February 7, 2021
published date: February 10, 2021
Citation: Malhotra P, Malhotra V, Gupta U, Paramjeet S Gill, Pushkar. “Epidemiological Profile and Clinical Spectrum of Hepatitis B-Ten Years’ Experience at Tertiary Care Centre Of Northeren India.’’. Gastroenterology and Surgical Gastroenterology, 2(1); DOI: http;//doi.org/03.2021/2.1007.
Copyright: © 2021 Parveen Malhotra. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly Cited.
Introduction: Hepatitis B virus (HBV) infection, a pan global health problem, has already effected one-third of the world population. India harbours around 40 million HBV carriers, thus accounting for 10–15% share of total pool of HBV carriers of the world. Every year over 100,000 Indians die due to illnesses related to HBV infection
Aims and objectives: To study the Epidemiological profile and Clinical spectrum of patients with Hepatitis B virus infection.
Materials & Methods: This prospective study was done at Department of Medical Gastroenterology, PGIMS,Rohtak over a period of ten years i.e. 01.09.2010 to 31.08.2020, on HbsAg positive patients who reported on outdoor patient department or were admitted in various wards of hospital.
Results: Hepatitis B is having certain hotspots in India like Haryana. The young males with rural background are most vulnerable because of unsafe needle & injection practices due to non-availability of proper health care facilities.
Introduction
Hepatitis B virus (HBV) infection, a pan global health problem has already effected one-third of the world population. Around 2 billion people have been infected worldwide and out of them, 350 million suffer from chronic HBV infection. In patients of HBV infection,15–40% of patients will develop cirrhosis, liver failure and hepatocellular carcinoma [1,4]. The prevalence of Hepatitis B surface antigen (HBsAg) is used to classify geographical areas as high (where > 8% of the population is HBsAg positive), intermediate (2–7%) or low (< 2%) HBV endemicity [5]. India harbours around 40 million HBV carriers,thus accounting for 10–15% share of total pool of
HBV carriers of the world. Every year over 100,000 Indians die due to illnesses related to HBV infection [6,7] and HBsAg positivity ranges between 2–4.7% [8-9]. In the natural history of HBV infection, the most important event is HBeAg seroconversion characterized by loss of HBeAg and development of antibody to HBeAg [10]. This generally occurs years after replicative phase and indicates transition to a low/non replicative phase with potential for resolution of infection and improvement of necro-inflammation in the liver. Age of acquisition of the virus, immune competence of the host and the strength of immune response to the viral antigens are some of the determinants of timing and efficiency of seroconversion. The prognosis of chronic HBV infection is dependent upon the amount of inflammation, necrosis and fibrosis in the liver at this point of seroconversion. If significant liver damage is already present at this point, then the prognosis after seroconversion, spontaneous or treatment related is unlikely to be good, despite suppression of viral replication. On the other hand, if the seroconversion had occurred early and is maintained, then the long-term prognosis is excellent. It has been shown that the probability of development of hepatocellular carcinoma is many fold higher in persons who are HBeAg positive, than who are only HBsAg positive and HBeAg negative. Since majority of infected people remain asymptomatic, and often present with advanced disease, early diagnosis is critical to timely initiation and scale up of treatment for viral hepatitis B. Inadequate public and health-care provider awareness; the asymptomatic nature of infection during the early stages, lifelong treatment and access to quality diagnostics are some of the challenges to scaling up management of viral hepatitis B. Routine assessment of HbsAg-positive persons is needed to guide HBV management and indicate the need for treatment. This generally includes assessment of measuring aminotransferase levels to help determine liver inflammation and stage of liver fibrosis by non-invasive tests (NITs) such as aspartate aminotransferase (AST) to-platelet ratio index (APRI). Serum HBV DNA levels/viral load quantified by real-time polymerase chain reaction (PCR) correlate with disease progression and are used for decisions to treat and subsequent monitoring. Antiviral agents active against HBV are available, and have been shown to suppress HBV replication, prevent progression to cirrhosis, and reduce the risk of HCC and liver-related deaths. However, currently available treatments fail to eradicate the virus in most of those treated, necessitating potentially lifelong treatment. Prevention strategies including needle exchange in people who injects drugs (PWID), barrier contraception need to be promoted in key affected populations, including persons who inject drugs, men who have sex with men (MSM), and sex workers; prevention of HBV transmission through immunization of health care workers need to be ensured in health-care settings. Voluntary blood donation and universal screening of blood and blood products for transfusion will also help in prevention strategies.
Review Of Literature
HBV infection prevalence in world ranges from 10% in some Asian and Western Pacific countries to under 0.5% in the United States and northern European countries. Most infections occur during infancy or childhood particularly acquired from the carrier mothers at birth. Since most infections in children are asymptomatic, there is little evidence of acute disease related to HBV but the rates of chronic liver disease and liver cancer in adults are high. The chronicity of infection depends upon age of acquering infection as more than 90% of the adults infected with HBV successfully clear the acute infection and become immune naturally. Approximately, 5-10% proceeds to chronicity and become chronic carriers. Whereas around 98% babies born to mothers with chronic HBV infection become infected and around 95% of these will develop a persistent infection. The reason behind this is that immune system of very young is less able to clear the infected hepatocytes. All pregnant women with HBV should be evaluated for the need of treatment for hepatitis B and any associated liver disease, and given advice about prevention of transmission. Only a proportion of those with hepatitis B virus infection (pregnant or otherwise) need treatment . Hepatitis B in a pregnant woman is not a reason for considering termination of pregnancy. Similarly, the presence of HBV infection is not an indication for caesarean delivery, which should be based on obstetric indications only. Administration of hepatitis B vaccine to pregnant women with HBV provides no benefit either to the mother or the baby. All infants born to HBV positive women need to be immunized within 24 hours of birth (Dose - 0) followed by 6, 10 & 14 weeks (dose – 10 µg IM) and HBIG – (0.5 ml or 100 international units, intramuscular), this should be done as soon after birth as possible (and within 12-24 hours) and in a limb other than the one in which hepatitis B vaccine has been administered.
Chronic HBV infection is a dynamic process reflecting the interaction between HBV replication, hepatocytes and the host’s immune response. The natural history of chronic HBV infection has been schematically divided into phases, taking into account the presence of HbeAg, HBV DNA levels, alanine aminotransferase (ALT) values and eventually the presence or absence of liver inflammation. The risk of progression to cirrhosis and HCC is variable and is affected by the host’s immune response. The natural history of HBV infection consists of five phases: the immunotolerant phase, the immune reactive HBeAg positive phase, the inactive HBV carrier phase, the HBeAg negative chronic hepatitis B phase and the HBsAg negative phase or resolution phase (11). Patients with HBsAg positivity presenting with jaundice may be due to acute HBV infection, superadded infections like hepatitis A or E or due to reactivation of the HBV virus. Cirrhosis, hepatocellular carcinoma and liver failure are major long-term complications of chronic HBV infection that significantly increases morbidity and mortality. In patients without cirrhosis, if untreated, the incidence of liver related death is low and ranges from 0 to 1.06 per 100 person years. The mortality rate at 5 years is 16% for those with compensated cirrhosis and is 65% to 86% for decompensated cirrhosis [12,13]. Various factors involving the host and the virus may contribute to the development of hepatocellular carcinoma. HCC incidence is three to six times higher in males than in females, suggesting a tumorigenic effect of androgens [14]. The older age (>45 years), a first degree relative with HCC, the presence of cirrhosis, and reversion activity are all thought to contribute to HCC development [14]. Patients should be considered for treatment when they have evidence of disease activity (serum ALT levels above the upper limit of normal (ULN) and/or liver biopsy showing moderate to severe active necroinflammation and/or at least moderate fibrosis along with evidence of viral replication with HBV DNA levels above 2,000 IU/mL. Patients with cirrhosis or liver or obvious evidence of active chronic hepatitis B with ALT above 2 times ULN and serum HBV DNA above 20,000 IU/mL may start treatment even without a liver biopsy. The risk of HBV infection may be higher in HIV-infected adults, and therefore all persons newly diagnosed with HIV should be screened for HbsAg and immunized if HbsAg is negative. Those already infected with HBV (HbsAg positive) do not benefit from HBV vaccine.
Aim Of Study
To study the Epidemiological profile and Spectrum of patients with Hepatitis B virus infection.
Material And Methods
This was prospective study done at Department of Medical Gastroenterology, PGIMS,Rohtak over a period of ten years i.e. 01.09.2010 to 31.08.2020,of patients who were found to be HbsAg positive on outdoor or indoor basis and were admitted in various wards of our hospital.
Inclusion Criteria
Patients who were found HbsAg positive on rapid card test or Enzyme linked immunoassay test and confirmed on Polymerase Chain test for HBV DNA quantitative test.
Exclusion Criteria
Patients who refused to give consent for enrollment in the study.
Methodology
In this prospective study, HBV patients who visited the Medical Gastroenterology Department in last ten years, and consented for enrollment in the study, their records were collected regarding their epidemiological profile and clinical spectrum. At inclusion time, detailed history of the patient was recorded like age, gender , residence, when hepatitis B detected , past history of any blood transfusion, surgery, needle stick injury, dental procedure, tattooing, acupuncture, unprotected intercourse with multiple sexual partners, intravenous drug abuse, history of previous upper GI bleed, hepatic encephalopathy, melena, history of other co morbidities like diabetes, hypertension, HIV, hepatitis C, chronic kidney disease, thyroid dysfunction. After that detailed clinical examination was done which included measurment of height, weight, BMI , complete general examination , tattoo, needle or puncture marks, icterus, stigmata of liver disease, organomegaly, ascites and systemic examination was done. The laboratory investigations were done like HBV DNA Quantitative, HBsAg, HBeAg, anti HBc IgM and IgG, anti HBeAg, anti HIV antibody, anti HCV antibody, complete blood counts, liver function tests , kidney function tests, serum electrolytes, coagulation parameters (PT, INR), blood sugar, ultrasonogram abdomen, chest x ray PA view ,ascitic fluid - TLC, DLC, cultures, SAAG, Upper GI endoscopy , CECT abdomen or Triple phase CT scan of abdomen and Fibro scan.
Stastical Analysis
Statistical analysis was performed by the SPSS program version 25.0. Continuous variables were presented as mean ± SD or median (range), and categorical variables were presented as absolute numbers and percentage. Data was checked for normality before statistical analysis using Shaipro Wilk test. Normally distributed continuous variables were compared using Student’s t test or ANOVA with appropriate post hoc tests. Categorical variables were analyzed using the chi square test. For all statistical tests, a p value less than 0.05 was considered to be significant.
Observation
The age distribution varied between 1-100 yrs of age and characterstically showed predominance of patients between 20-50 yrs of age group (65% of total patient pool) with highest peak in 20-30yrs of age ( 1468 patients i.e.30%). The number of patients were fewer in number at extremes of ages like from 0-10 yrs of age, only 39 patients i.e. 1%, from 10-20 yrs of age, 580 patients i.e. 12%, 80-90 yrs of age, 12 patients i.e.0.002% & 91-100 yrs of age, 4 patients i.e. 0.0008% contributed to total pool of 4828 hepatitis B patients.
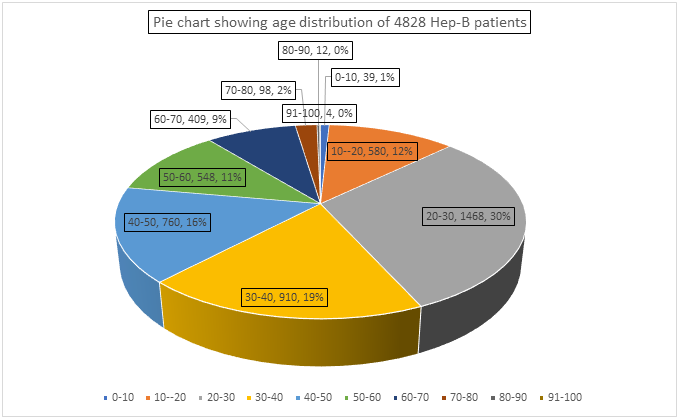
Figure 1: Showing Age Distribution of Hepatitis B patients
On analysis of clinical stages of diseases, maximum number of hepatitis B patients were chronic hepatitis B but in inactive carrier state i.e. 3646 patients (76%) who did not require any treatment. In total 700 patients (14%) of chronic hepatitis B were found to be in active phase and were started on treatment. The acute hepatitis B state was seen in 482 patients i.e. 10% of total patients of hepatitis B.
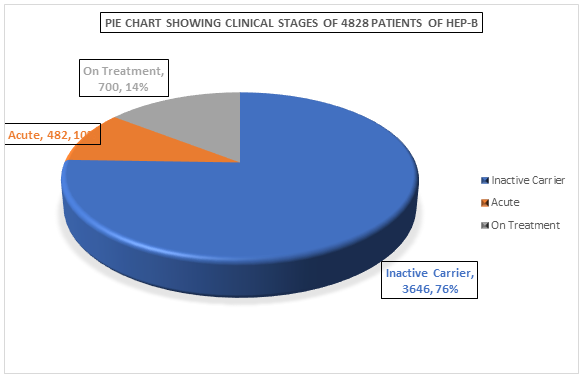
Figure 2: Showing Clinical Stages of Hepatitis B patients
When sex distribution was analyzed then strikingly 3224 patients (67%) were males and only 1604 patients (33%) were females.
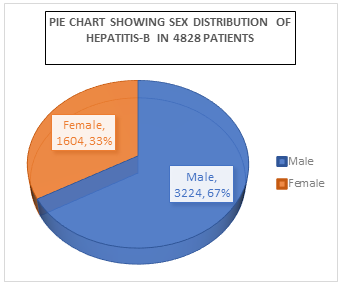
Figure 3: Showing Sex Distribution of Hepatitis B patients
Discussion
India is in intermediate zone of HBV prevalence and thus it is a major public health problem in India. About 40 million asymptomatic hepatitis B virus (HBV) carriers are present in India. There are no large-scale population studies of the prevalence of HBV in India. The smaller studies done show wide geographic variations due to differences in socio-economic status or cultural practices in different regions. The overall rate of HBsAg positivity has been reported to range between 2% and 8% in most studies [15-17]. The same fact has been confirmed in study by Malhotra etal,2020 in which retrospective analysis of five years, the yearly prevalence of HbsAg ranged between 3.16%- 8.1% with mean of 5.23% [18]. The widely quoted figure of a carrier rate in India of 4.7% with an estimated carrier population of 56.5 million.
Most of the available data is based on blood bank screening which does not truly reflect the national prevalence, as shown by Malhotra etal ,2020, in which blood bank data revealed prevalence rates of HBsAg and anti-HCV antibody positivity of 0.80% and 0.81%, respectively, whereas the rates of the same derived from passive screening data were 5.23% and 5.18%, respectively [19]. Many of the blood banks show HBsAg prevalence was 0.2–4%, most of which have prevalence much lower than that of the commonly quoted prevalence data [20]. This fact is also confirmed in our study, as out of total 4828 patients, only 148 patients (3.06% ) were detected during blood donation.
A large study involving 8575 pregnant women from Northern India, documented HBsAg carrier rate in antenatal mothers to be 3.7%, HBeAg carrier rate 7.8% and vertical transmission was observed in 18.6% [21]. However, a recent study by Dwivedi et al [22] has shown a lower prevalence rate of 0.9 In two different studies, Malhotra etal showed seroprevalence of HbsAg to be 0.34% in pregnant women [23,24]. In our present study out of total pool of 4828 patients, 70 pregnant females were found positive for HbsAg.
The peaking of infection rates in adulthood in Indian population also suggests a close relationship of acquisition of infection in the adults [25]. The prevalence of HbsAg was 2.97% in study conducted by Chaudhary A,2004 and there was a peak of prevalence after the second decade of life [26]. In our study also, the age distribution varied between 1-100 yrs of age and characterstically showed predominance of patients between 20-50 yrs of age group (65% of total patient pool) with highest peak in 20-30yrs of age ( 1468 patients i.e. 30%).
In an earlier study, frequent exposure to percutaneous injuries, repeated use of Parenteral injections for trivial illnesses and the untrained para-medical personnel, lacking in knowledge about modes of sterilization in primary care centers have been found to be the major factors that facilitate transmission of HBV, as well as other viruses in this population [25]. In our study also, 40% (1931) patients gave history of Parenteral injections, dental or any other surgical interventions & tattooing which reemphasizes the significant contribution of unsafe injection practices in spreading transmission of hepatitis B in the community. Moreover, 70% (3380 patients) of patients in our group of study came from rural background with lower socioeconomic status and as expected circumstantially they were exposed maximum to unsafe needle practices. Apart from exposure from extraneous sources, interfamilial aggregation of HBV infected persons in a family has been well documented in India [27]. HbsAg contamination of surfaces is widespread in homes of chronically infected persons [28]which may explain the non-sexual interpersonal spread of HBV such as among household contacts. Household contacts of subjects with chronic HBV infection are known to be at high risk of acquiring infection through multiple modes [29].
One thing which was characteristically observed in our study group was regarding certain districts of our Haryana state like Jind, Kaithal, Panipat, Karnal, Sonepat were found to be hotspots for hepatitis B, as maximum number of patients belonged to these districts. Surprisingly, all these districts are also hotspots for Chronic Hepatitis C, as reported by Malhotra etal in 2016 [30]. The reason can be that both hepatitis B & C are having common route of transmission i.e. blood borne and non-availability of proper health infrastructure thus leading to unsafe needle practices can be significant contributory factor for making these districts hotspots for both hepatitis B & C.
Adult patients with HBeAg-positive chronic hepatitis usually present in the third or fourth decade of life and are more frequently males [31]. In our study also there was male predominance strikingly 3224 patients (67%) were males and only 1604 patients (33%) were females. The spectrum of liver damage ranges from mild (approximately 20 to 40%) to moderate or severe chronic hepatitis (approximately 40 to 60%) or active cirrhosis (approximately 10 to 25%) [32]. In about two-thirds of the disease burden in India, it is represented by hepatitis e−ve disease, with low or undetectable viral load which naturally mitigates the disease severity to some extent [33]. In an another study, a significant number of patients were in the inactive/immunotolerant phase not requiring treatment [34]. The same findings were observed in our study as maximum number of hepatitis B patients were chronic hepatitis B but in inactive carrier state i.e. 3646 patients (76%) who did not require any treatment. In total 700 patients (14%) of chronic hepatitis B were found to be in active phase or cirrhotics and were started on treatment. In total pool of 4828 patients, only 71 patients (1.46%) were found to be cirrhotic and we analyze in treatment group, then out of total 700 patients which required treatment, the contribution of these 71 patients is around 10.14% only. This is positive outcome of early detection of hepatitis B infection by various inetiatives like screening camps in hotspots, screening before surgical interventions, dialysis, thalassemiacs, pregnancy etc. and early treatment if indicated, leading to less chances of progression to cirrhotic stage. In only 10 patients (0.20%) out of total 4828 patients developed hepatocellular carcinoma (H.C.C). Out of these ten patients, eight had cirrhosis and two were non-cirrhotic which emphasizes the fact that hepatitis B virus can directly proceed to H.C.C without passing through cirrhotic stage but in very limited number of patients. The acute hepatitis B state was seen in 482 patients i.e. 10% of total patients of hepatitis B.
In hepatitis B virus (HBV) or hepatitis C virus (HCV) endemic countries, patients are exposed to the risk of being co-infected with both viruses. Parenteral viral transmission could also lead to HCV/HBV co-infection. In patients infected with both HCV and HBV, the risk of developing liver cirrhosis (LC) and hepatocellularcarcinoma (HCC) is usually highert han those with mono-infectionof either virus [35-37]. Therefore, patients co-infected with hepatitis C and B require regular monitoring and aggressive antiviral treatment. In our study, out of total 4828 patients, HBV & HCV co-nfection was seen only in 68 patients (1.40%) whereas HBV & HIV co-infection was seen only in 19 patients (0.39%). The characterstic point was that majority of patients of co-infection belonged to hotspots which can be due to common mode of transmission i.e. blood borne. The very minimal percentage of co-infection in such a large group of 4828 patients can be explained on basis of one virus not allowing the survival of another virus in human body which is already well documented in literature.
Results
Hepatitis B is a major health issue in India with non-uniform distribution and certain geographical areas are hotspots like Haryana. The young males belonging to rural background are most vulnerable. The unsafe needle & injection practices along with vertical transmission are major reason for transmission of this disease. We cannot rely on blood bank data for determining the prevalence of it but screening of high risk population in atleast hotspots will reflect true picture. Moreover,it will lead to early detection of cases and thus will substantially decrease the development of long term complications like cirrhosis and hepatocellular carcinoma, as shown in our this study.