Gastroenterology and Hepatology Research
OPEN ACCESS | Volume 7 - Issue 1 - 2026
ISSN No: 2836-2888 | Journal DOI: 10.61148/2836-2888/GHR
Anthony Kodzo-Grey Venyo.
MB CHB FRCSED FRCSI FGCS UROL. LLM.North Manchester General Hospital, Delaunays Road, Manchester, M8 5RB. United Kingdom.
*Corresponding author: Anthony Kodzo-Grey Venyo, MB CHB FRCSED FRCSI FGCS UROL. LLM.North Manchester General Hospital, Delaunays Road, Manchester, M8 5RB. United Kingdom.
Received date: June 21, 2022
Accepted date: August 05, 2022
published date: September 26, 2022
Citation: Anthony Kodzo-Grey Venyo. (2022) “Shigella Infections of the Kidney and Urinary Tract: A Review and Update”, J of Gastroenterology and Hepatology Research, 3(2); DOI: http;//doi.org/09.2022/2.10133
Copyright: © 2022 Anthony Kodzo-Grey Venyo. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly Cited.
Urinary tract infections and infections of the kidney are common globally. Nevertheless, infections of the kidney and urinary tract that are caused by Shigella organisms are very rare and many clinicians would not have encountered Shigella infections of the kidney and urinary tract during their training and their clinical practices before and it would be envisaged that many clinicians globally would tend not to be familiar with the manifestations, diagnosis and treatment of Shigella infections of the kidney and urinary tract. Shigella infections most often tend to afflict the gastrointestinal tract. The ensuing article has illustrated that even though rare, Shigella infections of the kidney can infect the kidney and urinary tract. Shigella infections of the kidney and urinary tract could affect children as well as adults and these infections could be asymptomatic or they may be associated with non-specific symptoms which tend to be associated with the more common types of urinary tract infections and kidney infections. There may or may not be a history of prior or contemporaneous diarrhoea or gastrointestinal symptoms associated with Shigella infection of the gastrointestinal tract. Shigella urinary tract infections may present with lower urinary tract symptoms of urinary frequency, dysuria, fever, lethargy or felling unwell. Shigella infections of the kidney may present with loin pain, fever, and lower urinary tract symptoms. These symptoms are non-specific but in the scenario of an antecedent treatment for Shigella infection of the gastrointestinal tract, clinicians should have a high index of suspicion for Shigella infections of the urinary tract and kidney in children and adults. Diagnosis of Shigella infection of the urinary tract would generally be established based upon urine culture and sensitivity tests and blood culture in some cases as well as in the scenario of renal abscess, a positive culture of Shigella organism obtained from pus taken from the kidney would be required. Successful management of Shigella urinary tract infections of the urinary tract would include utilization of the most appropriate antibiotics taking into consideration the antibiotic sensitivity of the cultured Shigella organism in addition to supportive treatment to improve the general condition of each patient. Shigella infections tend to be common in developing countries but because of global traveling Shigella infection could be diagnosed anywhere in the world. Apart from routine haematology and biochemistry as well as blood culture which tend to be undertaken in cases of Shigella infections of the urinary tract and kidneys, radiology imaging of the renal tract is important ascertaining if a patient who has Shigella urinary tract infection or infection of the kidney has developed an abscess or not. Some of the risk factors associated with the development of Shigella infection include the following: Children who are younger than 5 years of age within low / middle income countries; Immunocompromised hosts including advanced age and malnourishment; Institutional environments: day care setting or nursing home; Developing areas that do lack facilities for running water or indoor plumbing. A high-index of suspicion for Shigella infection of the urinary tract and kidneys is required to ensure microbiology tests including urine culture is required to establish Shigella infection of the urinary tract and kidney. Empirical treatment without a bacteriology result would not confirm the diagnosis of Shigella infection. Knowledge of a previous Shigella infection of the gastrointestinal tract should enable a clinician to have a high index of suspicion for the possibility of Shigella infection of the kidney and urinary tract if a patient presents with urinary tract and loin symptoms.
Introduction:
It has been iterated that urinary tract infection (UTI) annually does tend to affect about 150 million people globally [1] [2] It has also been stated that even though other microorganisms had been reported to cause UTI, it is mostly caused by bacteria and it usually has tended to be treated with antibiotics. [1] [3] Some of commonest documented uropathogens do include: Escherichia coli (E coli), Klebsiella pneumoniae, Proteus Mirabilis, Enterococcus sp, Staphylococcus saprophyticus, and Pseudomonas aeruginosa [4] [5] UTI caused by Shigella sp. have been identified in rare occasions [6] [7] [8] [9] Shigella infections tend to involve the gastrointestinal tract, and in view of this, it would be envisaged that majority of clinicians would tend to be familiar with the manifestations of Shigella infections of the gastrointestinal tract as well as majority of clinicians globally would not have encountered a single case of Shigella infections of the urinary tract before during their training as well as during their clinical practices and therefore, there is the possibility that majority of clinicians globally would not be aware of the manifestations of Shigella infections of the urinary tract, their diagnosis, as well as their treatment. The ensuing article contains a review and update of Shigella infections in general and more detailed discussions related to the manifestations, diagnosis and treatment of Shigella infections of the urinary tract. This article is divided into two parts: (A) Overview which has discussed Shigella infections generally including some aspects of Shigella infections of the urinary tract and (B) Miscellaneous Narrations and Discussions from some Case Reports, Case Series, and some Studies Related to Shigella infections of the urinary tract.
Methods:
Various internet data bases were searched including: Google, Google Scholar, and PUBMED. The search words that were used included: Shigella infections, Shigella infections of the urinary tract, Shigellosis of the urinary tract, Shigella infections of the kidney, Shigellosis of the Kidney, Shigella infection of the urinary bladder, Shigellosis of the urinary bladder, Shigella infections of the prostate gland, Shigellosis of the prostate gland, Shigella infection of the testis and epididymis, Shigellosis of testis and epididymis. Sixty-two, (62) references were identified which were used to write the review and update of the literature which has been divided into two parts: (A) Overview which has discussed Shigella infections generally including some aspects of Shigella infections of the urinary tract and (B) Miscellaneous Narrations and Discussions from some Case Reports, Case Series, and some Studies Related to Shigella infections of the urinary tract.
Results:
[A] Overview
Definition / general statements [10]
It has been iterated that with regard to taxonomy, Shigella does belong to the order of Enterobacterales, family Enterobacteriaceae and that Shigella spp. and Escherichia coli do represent a single genomospecies but they are classified as separate genera for medical purposes due to distinctive disease [10]
It has also been iterated that Shigella, is a highly infectious, virulent gram-negative bacillus which does tend to cause diarrheal diseases, with progression to dysentery. [10]
Essential features:
With regard to the essential features of Shigella organisms, it has been iterated that Shigella organisms, are Gram negative bacilli which easily spread by means of the faecal-oral route of transmission and they tend to be highly infectious in view of their low infectious dose [10]
It has been documented that Shigellosis (Shigella infection) typically tends to manifest as self-limiting watery or bloody diarrhoea; nevertheless, Shigellosis could also cause severe disease, such as haemolytic uremic syndrome [10]
It has been iterated that it has tended to be difficult to distinguish Shigella from Escherichia Coli (E. coli) in view of high genomic and proteomic similarity between Shigella and E Coli. [10]
It has been documented that, children as well as immunocompromised individuals usually tend to have the highest risk of developing severe Shigella infections. [10]
Epidemiology:
With regard to epidemiology, it has been iterated that Shigella spp. Does comprise of 4 subgroups (species), which tend to infect human beings as the major reservoir; however, Shigella Spp, could also infect non-human primates: The sub-groups of Shigella Spp have been summated to include the ensuing: [10]
Group A: Shigella dysenteriae (S dysenteriae), which does comprise of 15 serotypes
Shigella dysenteriae (S. dysenteriae) type 1; which tend to cause severe infections of view of the production of Shiga toxin type 1 by the stx1 gene
Group B: Shigella flexneri (S. flexneri), which does comprise of 8 serotypes
Group C: Shigella boydii (S. boydii), which does comprise of 19 serotypes
Group D: Shigella Sonnei (S. sonnei), which does comprise of 1 serotype
Shigella sonnei (S. sonnei) disease has tended to be generally less severe
Shigella infection has generally tended to be transmitted by means of direct or indirect faecal-oral route
Direct contact with an infected individual or indirect transmission through food or water which had been contaminated with human faeces that contain Shigella spp.
Shigella spp, tends to be easily transmitted in view of the very low infectious dose
Less than 100 viable bacterial cells need to be ingested in order to cause infection, in part, due to their resistance to the low pH of the stomach
Shigella spp infection, tends to be associated with poor hygiene as well as poor sanitation
Utilization of chlorinated water could decrease the contamination rates of Shigella spp.
Globally, Shigella spp, has tended to be endemic within tropical and temperate climates; and reported cases of Shigella spp do represent only a small proportion of cases of infections. [10] The ensuing summations related to Shigella infections had been made: [10]
It has been iterated that 180 million human infections Shigella infections annually had been reported [11]
Only 1.5 million cases of Shigella infections annually had been reported within the developed countries
450,000 cases of Shigella infections had been documented to occur annually within the United States of America (USA).
1 million deaths related to Shigella infection had been documented to occur annually
Shigella sonnei (S. sonnei) which constitutes 77% of cases of Shigella infections, and Shigella flexneri (S. flexneri), have tended to be the first and second most common types of Shigella infections within the United States of America (USA).
Shigella flexneri (S. flexneri), Shigella dysenteriae (S. dysenteriae), and Shigella boydii (S. boydii) have tended to be: most common Shigella infections within the developing countries
Risk groups: The ensuing summations had been made with regard to the risk groups associated with the development of Shigella infections: [10]
Children who are younger than 5 years of age within low / middle income countries
Immunocompromised hosts
Advanced age
Malnourished individuals
Institutional environments: day care setting or nursing home
Developing areas which do lack facilities for running water or indoor plumbing
Risky sexual behaviour patterns
Individuals who have HLA B27 antigen tend to be predisposed to the development of post infection reactive arthritis
Immunity: [10]
There tends to be Little innate immune protection from Shigella infections
Convalescent antibody response to O antigen (LPS) had been postulated to offer type specific protection against the development of Shigella infections
Breastfeeding has been postulated to be protective for infants and young children
Prevention: Some of the summated ways for the prevention of Shigella infections include the ensuing: [10]
Proper hand hygiene as well as proper food handling
There has tended to be up to 40% Shigella attack rate within an infected household
Antibiotics could be utilized to reduce the length of shedding / transmission of Shigella infection.
There is no vaccine available for the prevention of Shigella infection.
Sites: With regard to the sites of Shigella infection, the ensuing iterations had been made: [10]
Gastrointestinal infections: Shigella infection of the gastrointestinal tract has tended to descends from distal small intestine to the large intestine (main site of infection)
Shigella infection has tended to be locally invasive; however, Shigella infection, rarely does penetrate beyond the lamina propria
Urinary tract infections: Shigella urinary tract infections are very rare and it would be envisaged that majority of clinicians globally would not have seen a case of Shigella infection of the urinary tract during their training and working lives.
Pathophysiology:
Salient points related to the pathophysiology of Shigella infections have been summated as follows: [10] One day to four days (1 - 4 day) incubation period tends to be required for Shigella infections to occur, but the incubation period could be up to 1 week. [12]
Shigella spp (Shigella spp.) does tend to harbour a large plasmid with virulence factors which do encode the proteins that are necessary to invade intestinal epithelial cells, including invasion plasmid antigens (IpaB, IpaC and IpaD) [10] [13]
Shigella organisms tend to bind M cells and they tend to invade the lamina propria, then invade enterocytes from the basolateral surface [14]
Lateral spread of Shigella infection to infect adjacent cells within intestinal epithelium tends to occur and the bacteria tend to utilize host cell actin for intercellular actin polymerization to propel into neighbouring cells, avoiding extracellular exposure
Invasion of macrophages does tend to induce cellular apoptosis as well as inflammation
Presence of white blood cells (WBCs) within the faeces does indicate that the patient has an inflammatory type of diarrhoea which is indicative of enteral-invasive bacteria or select protozoans.
Inflammation could emanate in ulceration which is a characteristic feature of shigellosis
Integrity of the intestinal epithelial barrier breaks down
The integrity of the intestinal mucosa does tend to impair the absorption of fluids as well as nutrients
Loss of fluids and intestinal bleeding causes bloody diarrhoea
Shigella dysentariae (Shigella dysenteriae) serotype 1 toxin (Shiga toxin) does tend to portend the ensuing features:
AB5 toxin with a catalytic A chain and 5 B chains do work by inhibiting protein synthesis
This does cause direct cellular damage to intestinal epithelium
This also does tend to cause Shigella associated complications, including haemolytic uremic syndrome (HUS) which could emanate in fatality.
This also does tend to drive cytotoxicity as well as the development of vascular lesions
Clinical features [10]:
The clinical features of Shigella infections tend to be mild and asymptomatic infections usually tend to be self-limiting, and they tend to last between 4 days to 7 days
Inflammatory diarrhoea, which is referred to as dysentery, and which is characterized by visible blood and white blood cells tend to be found within the stool
Often accompanied by abdominal pain and high fever
The development of diarrhoea does tend to cause dehydration and hyponatremia, which is attributable to severe electrolyte imbalance
As well as lethargy, and altered mental status
Shigella associated diarrhoea tends to be common in children
Rare complications or manifestation in immunocompromised individuals or patients who have chronic underlying conditions include the ensuing:
Bacteraemia
Perforation of the intestine
Ekiri syndrome which does emanate in:
Lethal / toxic encephalopathy
Seizures
Post Shigella-infectious manifestations tend to include the ensuing: [10], [15]
HUS, which is characterized by presence of anaemia, destruction of platelets as well as acute kidney injury (AKI).
HUS often tends to be associated with Shigella dysenteriae serotype 1 but it could be due to other Shiga toxin producing strains of Shigella or E. coli
Reactive arthritis / Reiter syndrome which is caused by Shigella flexneri
Erythema nodosum
Glomerulonephritis
Postinfectious inflammatory bowel syndrome (IBS)
Diagnosis of Shigella Infection [10]:
The diagnosis of Shigella infection tends to be non-specific or tends to be based upon suggestive diagnostic tests and following this it has been recommended to undertake additional microbiologic work up [13]
Faecal occult blood test in cases of Shigella infections of the bowel tends to be positive
Faecal leukocyte examination in Shigella infection of the bowel tends to be positive
Radiology imaging and colonoscopy tend to be undertaken in the investigation of cases of Shigella infection of the colon. [16]
Stool culture and isolate identification to rule out pathogens with similar growth [10]
Some of the documented stool culture and isolate identification tests that tend to be undertaken to exclude the differential diagnosis of Shigella infection and to confirm Shigella infection of the bowel include: [10], [12].
Hektoen enteric (HE) agar: this does demonstrate green colonies
Xylose lysine deoxycholate (XLD) agar: this does demonstrate red colonies
GN broth (BD) for enrichment
VITEK 2-gram negative ID card (BioMérieux)
Shiga toxin enzyme immunoassays (EIAs)
Stool gastrointestinal pathogen PCR multiplex panels testing tends to be undertaken and does tend to have the advantage of being faster in comparison with culture. [10], [17].
BioFire FilmArray Gastrointestinal Panel (BioMérieux)
BD MAX Enteric Bacterial Panel (BD)
Prodesse ProGastro SSCS Assay (Hologic)
NxTAG Gastrointestinal Pathogen Panel (Luminex)
Laboratory tests:
It has been iterated that Shigella spp., had been considered to be serologically defined biotypes of Escherichia coli (E. coli); but they have been classified as a separate genus for medical purposes, due to distinctive disease. [10]
Conventional matrix assisted laser desorption / ionization time of flight (MALDI TOF) mass spectrometry cannot be utilized to differentiate Shigella from Escherichia coli (E. coli): [10], [18].
Pathology examination of Shigella specimens does demonstrate Gram negative rod, nonmotile, facultative anaerobes
In cases of Shigella infections, there tends to be evidence of lactose non-fermentation upon MacConkey agar, which is consistent with some other diarrheal pathogens
Triple sugar iron (TSI) agar slant: glucose fermenter (acid producing at bottom), and there tends to be no fermentation of sucrose or lactose and use of amino acids (alkaline at top)
Expect: K / A, gas negative, hydrogen sulfide negative
IMVIC biochemicals: [10] [19] [20]
Indole: variable
Methyl red: positive
Voges-Proskauer: negative
Citrate: negative
It is worth realising that Shigellosis is a notifiable disease within the United States of America) (USA), and that local public health laboratories could undertake additional tests for epidemiologic purposes, including Shigella serotyping and antimicrobial susceptibility testing in order to follow resistance patterns of the disease
Treatment:
Treatment of Shigella infections of the bowel tends to be undertaken with utilization of a number of antibiotics as well as general management including the following: [10]
Rehydration the patient tends to be effective in shigellosis in which the stool volume is relatively low
Oral rehydration which is a low-cost treatment and which is easy to administer can be provided in some cases
Intravenous fluids could be utilized if the patient is vomiting or severely dehydrated
Antibiotic treatment tends to be given to patients who are severely ill [10]
It has been iterated that it is controversial if antibiotics should be utilized in mild cases of Shigella infections of the bowel who usually recover without antibiotics in 5 days to 7 days [10] [21]
Antibiotic treatment in Shigella infections of the bowel does tend to shorten the course of the Shigella disease and does reduce the time frame that organisms are shed
With regard to the treatment of Shigella infections of the bowel, clinicians have been advised always to refer to local antibiogram in order to ascertain the appropriate empiric antibiotics
Azithromycin
Azithromycin has tended to be the first-choice oral therapy for children
It has been pointed out that majority of clinical laboratories do not tend to test azithromycin susceptibility [10]
Ceftriaxone
It has been iterated that the first-choice parenteral therapy for children is Ceftriaxone. [10]
Ceftriaxone has tended to be utilized for the treatment of suspected / confirmed invasive Shigella disease. [10]
Utilization of Ceftriaxone has been documented to achieve high concentrations in serum and stool of patients who have Shigella infection of the bowel. [10] [22]
Ciprofloxacin
It has been pointed out that with regard to the treatment of Shigella infections of the bowel, the use of Ciprofloxacin antimicrobial resistance is increasing. [10]
Ciprofloxacin does tend to achieve high concentrations in serum and stool of patients who are treated for Shigella infection of the bowel with Ciprofloxacin.[10] [22]
Alternative treatments: Some of the documented alternative antibiotic treatments that had been utilized for the treatment of Shigella infection of the bowel include the following: trimethoprim sulfamethoxazole or ampicillin, if susceptible
It has been pointed out that with regard to the treatment of Shigella infections of the bowel, utilization of antidiarrheal medication is contraindicated [10] [11]
For example, loperamide, paregoric and diphenoxylate
And that utilization of anti-diarrhoeal agents does tend to delay the clearance of Shigella organisms
Utilization of anti-diarrhoeal for the treatment of Shigella infection of the bowel could prolong / worsen the symptoms of the patient
It has been iterated that utilization of antimicrobial treatment with utilization of ciprofloxacin and trimethoprim sulfamethoxazole could increase Stx production. [10] [23]
It has been iterated that the development of HUS could be characterized by various manifestations that could be treated with supportive treatment for example, for anaemia, thrombocytopenia, fluid and electrolyte disturbances, acute kidney injury and hypertension. [10]
Gross examination features:
Microscopy examination features of specimens of Shigella infected tissues have been stated to include: [10]
The tissues do upon macroscopy examination tend to be indistinguishable from other causes of ulcerative colitis
Acute onset of recto-sigmoidal lesions in cases of Shigella infections of the rectosigmoid region upon gross examination demonstrate the ensuing features: [14]
Erythema
Oedema
Focal haemorrhage and
Adherent layers of purulent exudate
The biopsy examination of specimens of bowel infected by Shigella does tend to demonstrate the following: [10], [14]
Oedematous tissue
Capillary congestion
Focal haemorrhage
Crypt hyperplasia
Goblet cell depletion
Mononuclear and polymorphonuclear cell infiltration
Shedding of epithelial cells and erythrocytes
Microulcerations
Molecular / cytogenetics features of tissues infected by Shigella:
It has been iterated that 16S rRNA gene sequencing will not differentiate Shigella spp. from E. coli due to greater than (>) 99% sequence similarity [10] [18]
It has also been iterated that molecular-assays that target virulence genes are successful for the detection of Shigella / entero-invasive Escherichia. coli and serotypes as summated below: [10]
Genes: ipaH, ipaB, ipaC, inv, ial
Primarily for epidemiology purposes rather than diagnostics
For example., BioFire Film-Array Gastrointestinal Panel uses gene targets to differentiate Shigella from E. coli, where Shigella is positive for ipaH and stx1
stx1 / stx2, can indicate Shiga-like toxin producing Escherichia. coli (STEC) or Shigella, this marker cannot distinguish alone
ipaH, invasion plasmid antigen detected in Shigella and entero-invasive E. coli (EIEC), this marker alone cannot distinguish the infecting organism
Differential diagnosis:
Some of the differential diagnoses of Shigella infections of the bowel had been summated as follows:
Invasive diarrhoeal disease – which tends to simulate diarrhoeal diseases that are caused Shigella could be differentiated by symptomatic multiplex molecular tests and the following characteristics: [10]
Entero-invasive Escherichia coli (E coli) EIEC: [10]
Also does encode for genes which allow for invasion of enteric epithelial cells
Majority of Escherichia coli (E. coli) isolates could be differentiated from Shigella based upon their motility and ability to ferment lactose
Shiga toxin producing Escherichia coli (E coli) (STEC): [10]
Also encodes for Shiga toxin
It has been stated that E. coli O157:H7 could often be identified on Sorbitol MacConkey (SMAC) agar as a non-sorbitol fermenter [24]
Salmonella: [10]
Hydrogen sulfide production upon TSI slant or XLD agar
Could also be recovered for other systemic sites
Campylobacter Jejuni: [10]
Growth at 42 °C in microaerophilic conditions
Could also be recovered for other systemic sites
Yersinia enterocolitis: [10]
Recovery and characteristic bullseye colony appearance with red center and pale border upon the Yersinia selective agar cefsulodin irgasan novobiocin (CIN) agar (BD)
Could also be recovered for other systemic sites
Entamoeba histolytica (E histolytica): [10]
E histolytica could be identified with utilization of a stool ova and parasite examination
Clostridioides difficile (Clostridium difficile). [10]
Strict anaerobe which could be identified through the detection of Clostridium difficile toxins A and B
Non-infectious diseases with chronic diarrhoea, including lactose intolerance, celiac disease, irritable bowel syndrome and ulcerative colitis will tend not to be associated with positive microbiologic findings [10]
Shigella Infections of the kidney and urinary tract infect:
Shigella infections of the kidney and urinary tract have been reported only on rare occasions and the symptoms have tended to be non-specific and a high index of suspicion is required to establish the diagnosis.
Shigella Infections of the Kidney
Some patients could be asymptomatic with or without a previous or contemporaneous history of diarrhoea or other gastrointestinal symptoms.
Some patients may have fever, loin pain, sensation of feeling unwell and with loss of appetite or urinary frequency, dysuria, and urinary urgency. These symptoms are non-specific and the diagnosis tends to be made based upon a urine culture finding of Shigella within the urine.
Shigella infections of the urinary tract.
Shigella infections of the urinary tract could affect children, who are males or females and it could also affect adult females and males.
Shigella infections of the urinary tract could be asymptomatic and the diagnosis would tend to be made based upon the urine culture and sensitivity results of routine specimen that has been sent for culture and sensitivity.
Some patients who have Shigella urinary tract infection would manifest with non-specific urinary tract infection symptoms of more common urinary tract infecting organisms including: urinary frequency, dysuria, fever, loin pain and other non-specific symptoms. There may or may not be any history of previous diarrhoea or gastrointestinal symptoms or contemporaneous gastrointestinal tract symptoms.
Diagnosis of Shigella infections of the kidney and urinary tract
The diagnosis tends to be made based upon culture of Shigella from urine specimens of individuals have had a history of Shigella infections of the gastrointestinal tract or some patients who have not had any previous history of Shigella infections of the gastrointestinal tract.
In cases of pus producing infections / pyonephrosis that is caused by Shigella, a culture of Shigella obtained from the kidney would confirm the diagnosis.
Laboratory studies:
Microbiology:
Urine tests
Urinalysis, urine microscopy and urine culture.
Urine microscopy and culture is a well-known way of establishing the cause of an unexpected Shigella infection of the urinary tract.
Pus culture
Pus that is obtained from the kidney for example in cases of pyonephrosis associated Shigella infection may grow Shigella organism in the absence of evidence of Shigella obtained from the stool.
Stool culture
Stool microscopy and culture tends to be undertaken in patients who have Shigella infections of the kidney or urinary tract to ascertain if the patients have contemporaneous Shigella infection of the gastrointestinal tract and kidney / urinary tract or the patients are also asymptomatic carriers of Shigella within their stool.
Haematology Blood tests:
Routine full blood count and INR tends to be a study that is undertaken for the general assessment of all patients who have Shigella infection of the kidney or urinary tract. The results would not be diagnostic but in case a patient is found to be anaemic, it would be investigated and treated to improve upon the general status of the individual.
Biochemistry blood tests.
Routine blood biochemistry tests including: CRP, EGFR, serum urea and electrolytes, liver function tests, bone profile and blood glucose tests tend to be undertaken to assess the general status of each patient and if there is any abnormality detected it would be investigated and treated accordingly to improve upon the general status of the patient whilst treating the patient for Shigella infection at the same time.
Blood culture also tends to be undertaken in patients who have symptoms and signs of severe urinary tract infections or kidney infections and at times the blood culture would tend to be positive in few cases.
Radiology investigations
Ultrasound scan
Ultrasound scan of the kidney and renal tract is a common radiology imaging investigation that tends to be undertaken to ascertain if there are any predisposing factors in all patients, whether they are children or adults, male or female, as well as if there are any abscesses that need to be treated.
Computed Tomography (CT) Scan and Magnetic Resonance Imaging (MRI) Scan
CT scan and MRI scan are other possible common radiology imaging options that tend to utilized for assessments of patients who have kidney infections or urinary tract infections to ascertain if there are any predisposing factors in all patients, whether they are children or adults, male or female, as well as if there are any abscesses that need to be treated.
Miscellaneous procedures
Micturating cystourethrogram, isotope renograms, cystoscopy and retrograde ureteropyelogram and uretero-renoscopy and miscellaneous studies that tend to be undertaken for selected patients as may be required based upon the predisposing factors of some individuals who have predisposing factors for the development of urinary tract / kidney infections that could be treated to reduce the incidence of recurrent infections.
[B] Miscellaneous Narrations, and Discussions from Some Case Reports, Case Series, And Studies Related to Shigella Infections of The Kidney and Urinary Tract:
Shigella Infections of the Urinary Tract and Kidney Problems:
Jao and Jackson [25] in 1963, iterated that Shigella dysentery is an infection in which the micro-organisms usually tend to be localized within the alimentary tract as well as within regional lymph nodes as well as that literature review; however, he identified that infection of the urinary tract as well as bacteraemia due to the Shigella species had been documented. Jao and Jackson [25] also stated that asymptomatic carriers of Shigella infection, had been recognized; however, they usually tend to follow acute bacillary dysentery. Jao and Jackson. [25] reported a 61-year-old Mexican who had asymptomatic urinary tract infection that was caused by Shigella sonnei who did not have a history of dysentery but was a faecal carrier of Shigella infection.
Narchi and Beattie [26] reported in 1987, the first case of asymptomatic bacteriuria due to Shigella sonnei in a girl who was aged 10 years and who did not have an antecedent history of dysentery or vulvovaginitis and who did not have any evidence of colonisation of the gastrointestinal tract. [26]
Park et al. [27] iterated that Shigella spp, does cause classic bacillary dysentery which rarely does result in extraintestinal complications. They also stated that urinary tract infections due to Shigella spp are not common and that Shigella sonnei urinary tract infections are very rare. Park et al. [27] reported a case of symptomatic urinary tract infection (UTI) due to Shigella sonnei in a 9-year-old girl who had manifested with a history of fever, abdominal pain, loose form diarrhoea, vomiting, and dysuria for 1 day. She had urine culture from which had identified Shigella sonnei was identified and within her stool culture there was no isolation of Salmonella or Shigella. She was treated with utilization of gentamicin and cefuroxime intravenously for a period of 5 days, which did control successfully the clinical features of the infection.
Ekwall et al. [28] reported a case of asymptomatic urinary tract infection which was caused by Shigella sonnei in a patient which they had iterated was the first case of asymptomatic urinary tract infection that was caused by Shigella sonnei. The patient was a 74-year-old man, who was noted not to be a faecal carrier of Shigella sonnei and who did not have any history of dysentery. He underwent treatment with utilization of pivmecillinam 400 mg three times per day for 14 days which did eradicate the bacteria. They additionally stated that the time and source of the infection was not known.
Anatoliotaki et al. [8] reported a case of urinary tract infection which was caused by Shigella sonnei in a 6-year-old girl who had vesico-ureteric reflux and a preceding history of gastroenteritis. The strain of Shigella sonnei was resistant to ampicillin and cotrimoxazole, and treatment with cefotaxime did eradicate the infection.
Awadalla et al. [7] reported a case of severe urinary tract infection which was caused by Shigella sonnei in a 3-year-old girl who had vesico-ureteric reflux and no history of dysentery. Treatment with co-trimoxazole in a dose of 48 mg/kg for 10 days was given and the infection was reported to be eradicated.
Papasian et al. [6] reported the clinical course for a patient who had symptomatic urinary tract infection due to Shigella sonnei. They also reviewed the role of Shigella spp. as urinary pathogens. Papasian et al. [6] iterated that DuPont [29] had documented that Shigella spp. usually tends to produce self-limited gastrointestinal infections which rarely result in extraintestinal complications [new 29]). Papasian et al. [6] additionally stated that Urinary tract infections (UTIs) due to Shigella spp. are not common, and it has been iterated that Shigella sonnei UTIs are particularly unusual [7] [25]. Papasian et al. [6] reported a case of symptomatic UTI due to S. sonnei. Papasian et al. [6] reported a 45-year-old female who had presented to an outpatient medicine clinic complaining of an 8-day history of fever and she stated that her temperatures taken at home peaked at 1028F [38.98C]), chills. She also manifested with left-flank tenderness, polyuria, dysuria, increased frequency of urination, nausea, occasional vomiting, epigastric pain, as well as diarrhoea which consisted of five to six loose stools per day. Her past medical history did consist of a right nephrectomy, polycystic kidney disease of her remaining kidney, appendicectomy, gastric bypass surgery, dumping syndrome secondary to gastric bypass, and lumbar diskectomy. Upon examination in the clinic, she was found to have a temperature of 99.8 degrees F (37.28C), a pulse of 60/min, a blood pressure of 112/70 mm Hg, and her respirations were 20 respirations per minute. She did exhibit left-flank tenderness, but there was absence of guarding and rebound tenderness on her abdominal examination and she had normal bowel sounds in all quadrants of her abdomen. The remainder of her physical examination was consistent with her past surgical history but her examination generally was otherwise unremarkable. Her Urinalysis demonstrated: a trace amounts of protein, 11 bacteria, 11 mucus threads, two to five erythrocytes per high-power field, and 25 to 50 leukocytes per high-power field; this urine specimen was also submitted for bacterial culture. She was admitted for evaluation to ascertain if she had pyelonephritis, and intravenous treatment with ceftriaxone (1 g every 24 h) was commenced. The results of significant laboratory data collected upon her admission were reported as follows: potassium, 3.1 mmol/litre; chloride, 110 mmol/litre; creatinine, 1.7 mg/dl; albumin, 3.0 g/dl; leukocyte count and differential, within normal limits. Her urine specimen which was collected in the clinic was inoculated onto eosin-methylene blue agar and trypticase-soy agar which contained 5% sheep erythrocytes (Remel, Lenexa, Kans.) by using a 0.001-ml quantitative urine loop. A gram-negative rod was identified in quantities of .105 CFU/ml on both media; the gram-negative rod was identified as S. sonnei by both an API 20E strip (bioMerieux Vitek, Inc., Rockland, Mass.) and a Breakpoint Combo plate 8 (Microscan, West Sacramento, Calif.). The organism had agglutinated within Shigella group D antiserum but it had failed to agglutinate in Alkalescens-Dispar and Shigella group A, B, and C antisera (Becton Dickinson Microbiology Systems, Cockeysville, Md.). As determined by breakpoint susceptibility testing (Breakpoint Combo plate 8; Microscan), the isolate was found to be susceptible to gentamicin, tobramycin, amoxicillin/clavulanate, ticarcillin-clavulanate, cefotetan, ceftriaxone, ceftazidime, imipenem, ciprofloxacin, nitrofurantoin, norfloxacin, tetracycline, and trimethoprim-sulfamethoxaxole; the organism was found to be resistant to ampicillin, piperacillin, and ampicillin-sulbactam. On the 4th day of her hospitalization, her stool sample was submitted for bacterial culture and examination for ova and parasites. Her stool for culture was inoculated into selenite broth and onto Hektoen, xylose-lysine-desoxycholate (XLD), Campylobacter-cefoperazone-vancomycin-amphotericin (CVA), and MacConkey-sorbitol agars (Remel). All of the agars with the exception of CVA were incubated for 48 hours at 358 Centigrade in 5% CO2 and 95% air; CVA was incubated at 428 Centigrade in a microaerophilic environment (Bio Bag type Cfj; Becton Dickinson Microbiology Systems) for 48 h. The selenite broth culture was incubated overnight at 358C in 5% CO2 and 95% air before being sub-cultured to Hektoen and XLD plates for an additional 24-hours incubation at 358 Centigrade in 5% CO2 and 95% air. The results of the examination for ova and parasites revealed negative results, and the stool culture was found to be negative for Salmonella, Shigella, and Campylobacter spp. and Escherichia coli O157:H7. The patient did have gradual resolution of all of her urinary symptoms but she continued to produce soft stools, which had been attributed to her gastrectomy-associated dumping syndrome. She was discharged after 5 days following her hospitalization; her antimicrobial treatment was switched at that time to amoxicillin-clavulanate (500 mg orally every 8 hours) for an additional 7 days.
Papasian et al. [6] made the ensuing summating discussions:
Even though Shigella spp. are highly communicable agents of bacterial diarrhoea and they have been noted for invasion of intestinal epithelial cells, it has been stated that Shigella rarely does produce extraintestinal infections: [25] [29]
Specimens other than stool samples from which Shigella spp. had been recovered do include the following: liver, mesenteric lymph nodes, cerebrospinal fluid, synovial fluid, vaginal lesions, lungs, conjunctival sacs, corneal scrapings, blood, cutaneous lesions of the penile shaft, and urine: [25] [29] [30] [31] [32]
UTI which has been caused by Shigella. Sonnei (S. Sonnei) is very rare; including their reported case, and they were aware of only seven reported cases in the literature. [7] [25] [26] [28]
Out of the seven reported cases of Shigella. Sonnei (S. Sonnei) UTI, three had occurred in children who were12 years old, five occurred in females, and four of the patients were asymptomatic, and two of the patients also had positive stool cultures.
The trends that were seen with S. sonnei UTIs were similar to those that were seen with infections by other Shigella spp.
A review of 40 reported cases of Shigella UTI (including S. sonnei infections) had demonstrated that 26 cases which amounted to 65% of the cases had occurred in females, 19 cases that amounted to 48% of the cases had occurred in children,12 years old, and all paediatric infections had occurred in females. [7] [25] [26] [28]
Majority of the patients which included 24 out of the 40 patients, had symptomatic UTI, but less than half of the patients had gastrointestinal symptoms which included16 patients out of the 40 patients, or positive stool cultures which were found 14 patients out of the 40 patients.
The commonest urine isolate was Shigella. Flexneri (S. Flexneri) which was isolated in 33 out of the 40 patients; nevertheless, most recently reported infections had involved Shigella. Sonnei (S. Sonnei).
The route by which Shigella spp. does gain access to the urinary tract had often been unclear.
It had been assumed that clinical infection or asymptomatic of Shigella carriage within the gastrointestinal tract does tend to provide a source for organisms which infect the urinary tract by an ascending retrograde route, particularly in females. [25]
Remia is another mechanism by which organisms might gain access to the urinary tract, but shigellemia has tended to be rare and it has been stated that Shigellemia is most likely to occur in neonates, malnourished children, and immunosuppressed (notably AIDS) patients. [32] [33]
It has also been iterated that Sexual transmission is also a possibility. [30]
Their reported case was that of a 45-year-old female who had symptoms of acute pyelonephritis. The results of their patient’s urine cultures demonstrated S. sonnei, however, her stool cultures, which had not been collected until the patient had received nearly 4 days of intravenous antimicrobial treatment were negative.
Their patient had manifested with gastrointestinal symptoms together with urinary tract symptoms, however, the significance of these symptoms was confused by the symptomatology of acute pyelonephritis and her history of gastric bypass and dumping syndrome. Thus, the source of S. sonnei in their patient and the mechanism of spread to the urinary tract had remained unknown.
Cohen et al. [34] in 1996, stated that the purpose of their study was to explore the possibility of detecting antibodies to Shigella sonnei lipopolysaccharide (LPS) within urine after infection or vaccination. Cohen et al. [35 new 34] measured urinary immunoglobulin A (IgA) and IgG antibodies and specific IgA secretory protein against S. sonnei They measured LPS by enzyme-linked immunosorbent assay (ELISA), after adjustment for urine concentration. They defined a significant antibody level as one above a cut-off value which was calculated from the geometric mean12 standard deviations of urinary anti-Shigella. sonnei LPS levels in 43 healthy hepatitis B vaccinees (controls). Cohen et al. [34] summarized their results as follows:
Out of 11 culture-proven cases of Shigella. Sonnei shigellosis, at convalescence 9 cases that amounted to 82% had significantly elevated levels of urinary antibodies to the homologous LPS.
The Shigella sonnei conjugate vaccine, composed of Shigella. Sonnei O-specific polysaccharide covalently bound to recombinant exoprotein A of Pseudomonas aeruginosa, elicited a significant urine IgA or IgG anti-LPS response in 60% (6 of10), 56% (9 of 16), 43% (16 of 37), and 14% (3 of 21) of the volunteers at 2 weeks, 6 weeks, 6 months, and 12months after vaccination, respectively.
The specificity of the urine antibody response to Shigella sonnei LPS was documented by the total lack of response in subjects who had received parenteral-Shigella flexneri2a recombinant exoprotein A conjugate (69 urine samples) or meningococcal tetravalent control vaccines (4 urine samples).
All of the volunteers who had lacked a significant response to Shigella. Sonnei LPS in serum also had lacked such response within their urine samples.
Seventy-four percent of the volunteers who had a significant IgA or IgG anti-LPS response in serum at convalescence or 14 days pursuant to their vaccination did show similar response in urine.
The ratio of the titre of secretory protein bound to IgA anti-S. sonnei LPS in urine to that in serum was 303 times higher than the ratio of anti-S. sonnei LPS total IgA titre in urine to that in serum, indicating that the urine IgA is of secretory origin.
The aforementioned findings did suggest the possible use of urinary Shigella LPS antibodies as markers of systemic and secretory immune responses following natural infection or vaccination.
At this stage, because of its limited sensitivity, the detection / identification by ELISA of Shigella LPS antibodies in urine cannot replace the same assay in serum as a definitive test in an individual with a negative result.
Natural Shigella infection does induce a mucosal and systemic immune response to Shigella antigens.
Passive hemagglutination and enzyme-linked immunosorbent assay (ELISA) had been employed for the detection of serum antibodies of various immunoglobulin classes which were developed against Shigella serogroup-specific lipopolysaccharide (LPS). [35] [36]
It had been reported that the rise in serum immunoglobulin A (IgA) to Shigella LPS does indicate recent infection with the homologous organism while IgG-specific antibodies are markers of more distant exposure to Shigella strains (5). [new 36 new 35]
A strong correlation between pre-existing IgG anti-LPS serum antibodies and acquired natural immunity against shigellosis had been demonstrated previously. [37]
In immunogenicity studies of candidate Shigella vaccines, a significant rise in serum antibodies to homologous LPS was utilized as evidence for stimulation of immunocompetent cells. [38] [39] [40]
A few studies had demonstrated that antibodies to various bacterial antigens could be detected in urine after natural infection of the urinary tract or other mucosal sites, or after vaccination. [41] [42] [43] [44] [45] [46] and that at lest part of these antibodies, are of mucosal origin. [43] [45]
There was no report on any attempt to measure urinary antibodies to Shigella antigens following natural infection or vaccination.
They had assumed, if anti-Shigella anti-bodies could be detected within urine at high sensitivity and specificity, they could have the same applications as the serum anti-Shigella antibodies.
Urine samples could replace blood samples among young children for whom vein punctures tend to be problematic, and furthermore, the urinary antibodies may be utilized as reliable and direct markers of mucosal stimulation.
In their reported, they had examined the possibility of detecting anti-Shigella LPS antibodies in urine by a simple ELISA system following Shigella natural infection or parenteral vaccination with the Shigella sonnei-recombinant exoprotein A (rEPA) conjugate vaccine.
They also studied the nature of the urinary anti-bodies and the correlation between the levels of these antibodies and those of serum antibodies against the same LPS antigens.
Al-Soub et al. [47] stated the following:
Perinephric abscess, which is a collection of purulent material within the space between the kidney and Gerota’s fascia, is a relatively uncommon, life-threatening but treatable disease. [48]
The commonest route of infection is direct spread from the urinary tract, in which there usually tends to be an underlying parenchymatous disease. [48].
The source of infection could also be blood-borne. [50].
On rare occasions, a perinephric abscess does tend to emanate from gastrointestinal pathology. [51]
The microbiology of perinephric abscess is broad including the ensuing organisms: Escherichia coli, Proteus species, and Staphylococcus aureus are the most common etiologic agents. [50].
Perinephric abscess due to Shigella spp had not been reported before.
Surgical complications of shigellosis which had been reported in the English language literature include appendicitis with or without perforation, colonic perforation, intestinal obstruction, peritonitis, and intraabdominal abscess. [52]
They had reported a case of Shigella flexneri perinephric abscess and review the pertinent literature.
Al-Soub et al. [47] reported 70-year-old man who patient was admitted to Hamad Medical Corporation in June 2002, and who had manifested with right-sided abdominal pain, vomiting, as well as bloody diarrhoea of 4 days duration. His past history was noted to be unremarkable apart from the fact that he had recently been diagnosed with diabetes mellitus. His clinical examination demonstrated an ill-looking, restless, dehydrated patient who had a blood pressure of 60/40 mm Hg, a pulse rate of 120/minute, a temperature of 35.6°C, and a respiratory rate of 30/minute. His abdominal examination demonstrated generalized guarding, with marked tenderness and fullness within his right iliac fossa. He had a rectal examination which showed fresh blood. The results of his laboratory investigations demonstrated a white blood cell count (WBC) of 13 900/mm3 (95% segmented neutrophils, 3% lymphocytes, and 2% monocytes), haemoglobin 13.1 g/dL, platelet 238 000/mm3, serum creatinine 253 μmol/L, blood urea nitrogen 33.9 mmol/L, pH 7.22, pCO2 19.4 mm Hg, pO2 100 mm Hg, random blood sugar 17.3 mmol/L, lactic acid 1.9 mmol/L (normal, 0.5–2.2 mmol/L). International normalized ratio (INR) 1.7, fibrinogen level 7.55 g/L. His urine microscopy was normal except for the presence of red blood cells, and his urine culture was negative in that no organism was cultured. He had chest radiograph which demonstrated a raised right dome of the diaphragm, a few right basal atelectatic bands, as well as a small right-sided pleural effusion. He had computed tomography (CT) scan of his abdomen without contrast which demonstrated right perinephric abscess, with anterior displacement of the right kidney, and mild hydronephrosis in both kidneys (see figure 1). He also had ultrasound scan of the prostate gland which showed an enlarged prostate gland. Pursuant to his hydration with intravenous fluids and administration of intravenous ceftriaxone and insulin, he underwent surgical drainage of his perinephric abscess through a small incision within his right flank under local anaesthesia. About 150 mL of pus were drained. Cultures of the pus and blood yielded Shigella flexneri which was sensitive to ciprofloxacin and ceftriaxone. His stool culture three days after his admission was negative for Shigella flexneri. He had stool, microscopy examination, which was positive for occult blood but it was negative for parasites. Colonoscopy was planned but the patient refused to undergo colonoscopy. After the result of cultures were received, ceftriaxone was discontinued and intravenous ciprofloxacin was added to his treatment. The condition of the patient improved, with gradual improvement of his renal function to normal levels before he was discharged. Intravenous antibiotics were administered for a total of 21 days and then this was changed to oral ciprofloxacin. He had a repeat CT scan before his discharge which demonstrated almost complete resolution of the perinephric collection (see figure 2). He was discharged home on the thirty-third day of his hospital admission in a good condition on oral ciprofloxacin, which he took for another 10 days.
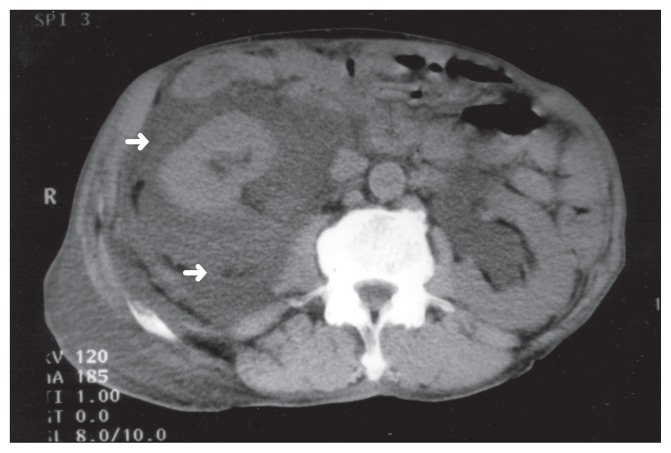
Figure 1: Computerized tomographic (CT) scan of the abdomen without contrast showing a large right perinephric collection with anterior displacement of the right kidney (arrows) associated with bilateral hydronephrosis.
Under Copy right: © 2005, Annals of Saudi Medicine. This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License
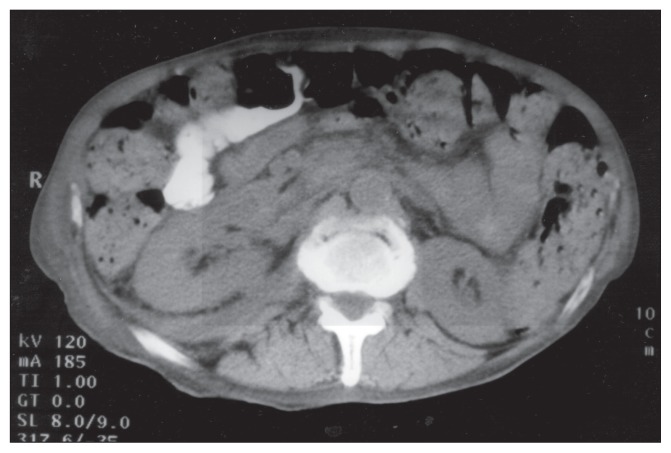
Figure 2: Follow-up CT scan of the abdomen with oral contrast showing almost complete resolution of the right perinephric collection.
Under Copy right: © 2005, Annals of Saudi Medicine. This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License
Al-Soub et al. [47] made the ensuing summations and discussions:
It has been documented that: infections that are caused by Shigella spp. usually tend to be self-limited and confined to the mucosa of the distal ileum and the colon. [52]
Even though Shigella SPPs, tend to be highly communicable agents of bacterial diarrhoea and they have been noted for invasion of the intestinal epithelial cells, they rarely do produce extraintestinal infections. [29]
It has been iterated that: specimens, other than stool samples from which Shigella spp. had been recovered do include those of the mesenteric lymph nodes, cerebrospinal fluid, synovial fluid, vaginal lesions, lungs, conjunctival sacs, corneal scrapings, blood, and cutaneous lesions of the penile shaft. [30]
Shigella spp. as a urinary pathogen had been occasionally reported. About 40 cases had been reported, manifesting either as urinary tract infection or asymptomatic bacteriuria. [6]
The route by which Shigella spp does can gain access to the urinary tract had often been unclear. It has been stated that with regards to Shigella SPP of the urinary tract it had been presumed that clinical infection or asymptomatic carriage within the gastrointestinal tract does provide a source for organisms which infect the urinary tract by the ascending retrograde route. [53]
It has been iterated that bacteraemia is another mechanism by which Shigella SPP might gain access to the urinary tract. [6]
Even though colonoscopy was not undertaken so as to definitely exclude colonic perforation with direct spread to the perinephric region, the computed tomography (CT) scan findings were not suggestive, and they believed that bacteraemia of colonic origin was the route through which the Shigella spp did reach the perinephric tissues in their patient.
Perinephric abscesses are a relatively rare condition, and it has been iterated that a large proportion of their mortality is the emanation of failure to diagnose the infection in a timely fashion. [48]
This failure to diagnose the infection may be because of the frequently obscure or nonspecific nature of the clinical manifestation of the infection. Diagnosis of this infection does require a high index of suspicion.
The commonest manifesting features include: fever, unilateral flank pain and tenderness. A flank or abdominal mass has tended to be present in less than 50% of patients.
Routine laboratory tests are non-specific. Urine and blood cultures had been positive in 60% and 40% of the Shigella infections respectively. [49]
It has been iterated that the key to establishing the diagnosis of Shigella infection with per-nephric abscess is to consider this entity in the differential diagnoses and to perform the appropriate radiology imaging studies in the form of ultrasound scan, CT scan, or magnetic resonance (MR) imaging. [49]
It has been iterated that the treatment does require both utilization of antibiotics and drainage, which has usually tended to undertaken percutaneously under CT or ultrasound guidance.
It has been stated that surgical drainage of Shigella peri-nephric abscess tends to be undertaken when percutaneous drainage fails or is contraindicated. [49]
The utilization of ceftriaxone initially in their treatment was to cover for organisms of the urinary tract origin, namely Enterobacteriaceae; nevertheless, when the culture results and sensitivity became available, it was changed to intravenous ciprofloxacin, which was changed to oral ciprofloxacin after the patient’s condition had improved.
The decision in their patient to drain the abscess surgically was made by the surgeon, who thought that the patient was very ill with a prolonged INR, and in order to ensure complete drainage of the abscess.
A large number of organisms had been reported to cause perinephric abscess, but the commonest organisms had been stated to include: Escherichia coli, Proteus species and Staphylococcus aureus. [50]
Among the organisms which had been rarely reported to cause perinephric abscess do include Salmonella spp. and Streptococcus pneumoniae. [49]
Perinephric abscess caused by Shigella spp had not been reported before.
The exact mechanism by which Shigella flexneri infected the perinephric tissues in their patient was uncertain. Nevertheless, considering the normal urine microscopy, the negative urine culture for Shigella flexneri and the patient’s symptoms, their patient probably had had bacillary dysentery, which had emanated in the development of bacteraemia and seeding of the perinephric tissues. Stool culture for Shigella flexneri was negative in their patient; nevertheless, this could have been due to the delay in the submission stool for culture for 3 days after commencing antibiotics.
Their patient did have many interesting features. It has been widely believed by both physicians and microbiologists that bacteraemia is a rare event in cases of shigellosis. It has been iterated that Shigellosis is particularly rare in adults and Shigellosis is mostly seen in children [54]. or in immunosuppressed individuals, particularly AIDS patients, and in the malnourished individuals. [6]
It has been iterated that Shigella bacteraemia often tends to be associated with a high mortality, with a case fatality rate of 46%. [55]
Another interesting feature in their patient was the fact that Shigella flexneri was the cause of his perinephric abscess. This association had never been reported before. They had undertaken a Medline search from 1966 to the time of publication of their article, and they did not find similar cases.
The reason for the lack of association is perhaps multifactorial, which does reflect the rarity of the condition and possibly underreporting of such a type of case in view of the fact that majority of cases of shigellosis do occur within poor countries where under- or no reporting has tended to be common.
Despite many unfavourable features in their patient, which did include old age, an acute manifestation with multisystem failure, and having a potentially fatal disease, he did have a good recovery, and he was discharged home ambulant with normal renal function.
Al-Soub et al. [47] made the ensuing conclusions:
Perinephric abscess is an uncommon complication of shigellosis.
Shigellosis abscess should be suspected in patients with perinephric abscess who have had a history suggestive of dysentery, especially in the absence of renal parenchymal or collecting system abnormality.
The management Shigellosis perinephric abscess should follow the same line of treatment of perinephric abscesses due to other organisms.
Early recognition and prompt drainage of the Shigellosis peri-nephric abscess, in combination with appropriate antibiotics, should reduce the morbidity and mortality that is associated with this condition.
Oh et al. [56] iterated that Shigella infection usually tends to produce gastrointestinal symptoms; however, it rare does cause urinary tract infection. Oh et al. [56] reported a 7-year-old girl who was admitted because she had fever, chills, right flank pain, as well as dysuria. She did not have any vomiting or diarrhoea. Upon examination, she was found to be tender within the right side of her lower abdomen, and right CVA tenderness. A clinical diagnosis of acute pyelonephritis was made based upon features of computed tomography (CT) scan of abdomen that she had undergone. She demonstrated improvement with administration of intravenous antibiotics. Her first urine culture grew Shigella dysenteri. Oh et al. [56] iterated that even though urinary tract infection due to Shigella species has tended to be very uncommon, Shigella species should be considered as a possible cause of paediatric urinary tract infection. Oh et al. [56] also iterated that they had reported the first case of urinary tract infection which had been caused by Shigella dysenteri, which manifested as acute pyelonephritis without gastrointestinal symptoms in a child.
Tufon et al. [1] reported a 2-years and 7 months-old-girl, who was admitted following persistent fever. They stated that an auto-medication with amoxycillin was reported. Urinalysis was undertaken on the first day and the sediment of her cloudy urine demonstrated many bacteria as well as few pus cells. Ceftriaxone was commenced as empirical therapy and a request for urine culture as well as blood culture was made. Three days following her admission, her temperature and CRP were 39.0 degrees centigrade and 96 mg/l respectively. Her urine culture grew Shigella flexneri that was sensitive to ofloxacin that was reported of the patient’s 4th day of admission. The patient was put on ofloxacin. Three days subsequently, the patient’s temperature which was 38.5 degrees centigrade and her CRP which was 24 mg /l, were still raised. Her blood culture did not grow any organism. A second urine culture was undertaken and this grew E coli that was resistant to ofloxacin and sensitive to meropenem and amikacin. Ofloxacin was stopped and she was commenced on meropenem and amikacin. Her third urine culture did not grow any organism after 48 hours of incubation. The patient was discharged looking well once more and her temperature was normal. Tufon et [1] made the following iterations:
Urinary tract infections (UTIs) tend mostly to be caused by bacteria.
Urine cultures usually tend to be a definitive measure to select the appropriate antibiotics for the elimination of a uropathogen as well as subsequent recovery from the infection.
Nevertheless, the preferred antibiotics as determined by urine culture and sensitivity, might still not eliminate the infection and would require further examination to establish the cause of failure of treatment which could be un-resolved bacteriuria, persistence of bacteria, immediate re-infection with a different uropathogen, or misdiagnosis.
Tufon et al. [1] made the following conclusions:
Antibiotics that is tailored towards the elimination of a particular bacterial species, could as well provide a favourable environment for other bacterial species which are resistant to it in the course of treating an episode of UTI.
This apparent failure of treatment could first of all require a second urine culture for the confirmation rather than considering the possibilities of misdiagnosis.
Karakas et al. [9] undertook a retrospective study, in order to evaluate 41,124 urine cultures which were studied within their microbiology laboratory between 2011 and March 2013, in order ascertain UTIs related to Shigella spp. They reported that the number of positive urine samples were 8421 which amounted to 20.4% of the urine samples and among these Shigella spp was isolated from six urine samples which amounted to 0.07% of the urine samples and four of these were Shigella boydii, and one was Shigella flexneri, as well as one was Shigella dysenteriae. There was no cluster to a certain time in that they were dispersed throughout the period of observation. All but one case which they called case 1 were outpatients. The blood culture was negative for case 1 and not studied for outpatient cases. In order to avoid stool contamination, stool samples were collected by the team from patients who had positive urine cultures, prior to the commencement of their treatment. In their patients including case 1, they did not isolate from the stool samples. The urine samples were obtained from patients with utilization of mid-stream clean catch technique, within different departments and cultured 5% sheep blood agar as well as Eosin Methylene Blue (EMB) agar plates and incubated overnight of over a period of 18 hours to 24 hours at 36 degrees centigrade. Lactose negative colonies which grew upon EMB agar were identified with utilization of biochemical tests (three sugar iron agar, urease, citrate, indole and they were confirmed by an automated system (Phoenix TM 100 BD, USA). They did confirm Shigella spp. isolates with utilization of specific antisera (BD Difco, USA). They tested susceptibility to ampicillin, cefotaxime, trimethoprim-sulfamethoxazole (TMP_SMX), nalidixic acid and ciprofloxacin by Kirby-Bauer disc diffusion method based upon the guidelines and breakpoints that had been established by the Clinical Laboratory and standards institute. [5 new 57] Institute CaLS. Performance Standards for antimicrobial They utilized E coli ATCC 25922-strain as the control of their study susceptibility testing; 22nd information supplement CLSIM100-S22. PA: Clinical and Laboratory Standards Institute Wayne; 2012]. Karakas et al. [9] made the ensuing iterations and discussions:
It is well known that Shigella SPPs., do cause gastrointestinal infections.
Extra-intestinal presentations of Shigella SPPs are not common and they mainly tend to include neurological presentations as well as arthritis. [29]
It has been iterated that urinary tract infections (UTIs) that are due to Shigella SPP, are rare / uncommon, and Shigella sonnei UTIS tend to be particularly unusual. [6]
S. sonnei could be responsible for UTI during pregnancy even when there are no predisposing factors or apparent source of infection identified. [58]
In a study from the United States of America (USA) laboratory had confirmed 208,368 Shigella isolates were examined and out of these, 71.7% were Shigella sonnei, 18.4% were Shigella flexneri, 1.6% were Shigella boydii, and 0.7% were Shigella dysenteriae. Nearly all of these strains that amounted to 99% were recovered from the stool and only 0.63% were from the urine. [59] This study also did stress that urine is the second commonest source of positive isolates after faeces.
To the best of their knowledge, there were no reported cases of urinary tract infections that had been related to Shigella boydii.
In a study that was undertaken between 2000 and 2010, Shiferaw et al. [60] had reported resistance in 1118 Shigella isolates to various antibiotics including: ampicillin 74%, streptomycin 58%, TMP_SMX 36%, tetracycline 28%, nalidixic acid 9%, as well as ciprofloxacin.
Another study which was undertaken within their hospital between 2001 and 2004 had demonstrated that resistance in 31 Shigella isolates were TMP-SMX 71%, tetracycline 71% and amoxycillin-clavulanat 9.7% as well as no resistance had been reported to ciprofloxacin and gentamicin. [61]
In their study, all Shigella associated with carcinoma of prostate was found in one. Diabetes mellitus is a well-known factor for the development of UTIs.
Shiferaw et al. [60] had reported that the incidence of Shigellosis was found to be highest among children who are aged between 1 year and 4 years as well as Shigella was more invasive especially with regard to children and elder ages.
In their study, the patients were all females, at elder ages and they had diabetes mellitus.
They found that having diabetes mellitus, being female, and elderly ages could have increased the risk for the development of Shigella UTI.
Karakas et al. [9] recommended that with regard to patients who have risk factors such as age and underlying diseases, UTI that is caused by Shigella should be kept in mind by having a high index of suspicion.
Baka et al. [58] reported a case of urinary tract infection (UTI) due to Shigella sonnei during pregnancy. Baka et al. [58] reported a 31-year-old pregnant woman was admitted complaining of left-flank tenderness, dysuria, and fever. Baka et al. [58] summated the results as follows: Following her examination and assessment, significant laboratory data were collected including increased leukocyte count of 10,800/ul with 86% neutrophils, and C-reactive protein of 9.6 mg/dl. Her urinalysis revealed 30 to 50 leukocytes per high power field while from the quantitative urine culture Shigella sonnei was cultured after 24 hours of incubation at 37 degrees C. After a two-week course of treatment with 750 mg cefuroxime every 8 h, the patient experienced gradual resolution of all of her symptoms and urinary cultures were negative two weeks and one month, respectively, pursuant to completion of her treatment. Her gestational course was uneventful and the patient delivered a healthy baby girl at term. Baka et al. [58] concluded that:
Shigella sonnei could be responsible for UTI during pregnancy even when no predisposing factors or an apparent source of infection could be identified.
John et al. [62] reported a case of urinary tract infection (UTI) due to Shigella sonnei in an adult woman who had diabetes mellitus. John et al. [62] reported a 64-year-old woman, who manifested with symptoms of UTI. Her urine culture grew- gram-negative bacilli with a significant colony count of >100,000 cfu /ml. Stool culture was also undertaken Shigella sonnei was isolated from her urine culture but was negative following 4 days of antibiotic treatment. She was known to new-onset diabetes mellitus. John et al. [62] made the following iterations:
Shigella species does belong to the family of Enterobacteriaceae which are causative agents for bacillary dysentery.
The entire spectrum of disease which is caused by Shigella species is referred to as Shigellosis.
Shigella does have four major subgroups including: Shigella dysenteriae, Shigella flexneri, Shigella boydii, as well as Shigella sonnei.
Shigella sonnei does tend to cause the mildest form of bacillary dysentery, and in many cases, it has tended to cause only mild diarrhoea.
Shigella sonnei, infection is the commonest Shigellosis within the developed countries.
There are few case reports of asymptomatic bacteriuria due to Shigella sonnei both in adults and in paediatric patients.
Few cases of symptomatic urinary tract infection (UTI) had been reported in the literature.
With regard to paediatric patients, the common risk-factor for the development of UTI had been found to be vesicoureteric reflux, especially with regard to females.
Recent studies that had been undertaken in various countries had demonstrated an alarming increase in the resistance of Shigella species to commonly utilized antibiotics including: chloramphenicol, ampicillin, co-trimoxazole, nalidixic acid, fluoroquinolenes, macrolides, as well as cephalosporins.
Many outbreaks of Shigellosis by multi-drug resistant (MDR) strains, had also ben reported.
Shigella does on rare occasions cause extra-intestinal manifestations including haemolytic uraemic syndrome, hyponatraemia, reactive arthritis, altered neurological state, bacteriaemia, urinary tract infection (UTI), and vulvovaginitis.
Urinary tract infection (UTI) is an uncommon complication of Shigella infection.
Taking into consideration the emergence of antibiotic resistance among Shigella sonnei isolates, having a knowledge of antibiotic sensitivity pattern of the Shigella organism is important in the treatment of such infections.
In their study, the isolate was not MDR. It was found to be sensitive to ampicillin, cefixime, cefotaxime, ciprofloxacin, nitrofurantoin, norfloxacin, azithromycin, and resistant to co-trimoxazole, as well as nalidixic acid.
Conclusions:
Shigella infections of the urinary tract and kidney are rare infections which could be encountered more commonly in the developing world but because of global travel these infections could be encountered anywhere in the world.
Shigella infections of the urinary tract and kidney could be asymptomatic or they may be associated with urinary tract symptoms alone or lower urinary tract symptoms and loin pain and fever which are non-specific symptoms.
In cases of Shigella infection of the urinary tract and Kidney, there may or may not be an antecedent or contemporaneous history of diarrhoea or gastrointestinal symptoms associated with Shigella infection.
Diagnosis of Shigella infection of the urinary tract and kidney does require a positive bacteriology culture of Shigella in the urine of the patient or of pus from the kidney.
It is important to be aware of the fact that a number of Shigella organisms from the urine could be resistant to some of the antibiotics that are used to treat the common causes of urinary tract infection.
A high-index of suspicion is required to establish an early diagnosis of Shigella infection of the urinary tract and kidney in order to establish quick and appropriate effective treatment for the infection.
Conflict of Interest – None
Acknowledgement
Acknowledgements to: Ann Saudi Med. for granting permission for reproduction of figures and contents of their journal article Under Copy right: © 2005, Annals of Saudi Medicine. This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License