Gastroenterology and Hepatology Research
OPEN ACCESS | Volume 7 - Issue 1 - 2026
ISSN No: 2836-2888 | Journal DOI: 10.61148/2836-2888/GHR
Anmol Mittal1*, Aaron Kahlam1, Alexander Le1, Dayna Panchal2 and Sushil Ahlawat2
1Department of Medicine, Rutgers New Jersey Medical School, Newark, New Jersey, USA
2Division of Gastroenterology and Hepatology, Department of Internal Medicine, Rutgers New Jersey Medical School, Newark, New Jersey, USA
*Corresponding author: Anmol Mittal, Department of Medicine, Rutgers New Jersey Medical School, Newark, New Jersey, USA.
Received date: June 15, 2022
Accepted date: June 21, 2022
published date: June 27, 2022
Citation: Anmol Mittal, Aaron Kahlam, Alexander Le, Dayna Panchal and Sushil Ahlawat. (2022) “Do Hospital Characters, Demographics, and Post-Procedure Complications Differ in Patients with Achalasia Undergoing Pneumatic Dilation versus Laparoscopic Heller’s Myotomy”, J of Gastroenterology and Hepatology Research, 3(2); DOI: http;//doi.org/06.2022/2.10132
Copyright: © 2022 Anmol Mittal. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly Cited.
Introduction:
Achalasia, an esophageal motility disorder, has an unknown etiology and does not have a curative treatment. Without treatment, patients with achalasia may go on to develop progressive dilation of the distal esophagus or even malignancy. Current techniques for disease modification include pneumatic dilation and laparoscopic Heller’s myotomy (LHM), as well as new emerging techniques including peroral endoscopic myotomy (POEM). The current accepted gold standard for treatment is a LHM, however, there have not been any established guidelines to decide between the treatment options for different types of patients with achalasia. As with all interventions, there are complications associated with the procedures. Our aim was to understand characteristics of admissions and patient demographics that may influence treatment decisions and post-procedure complication rates.
Methods:
A retrospective analysis of the Nationwide Inpatient Sample (NIS) 2001-2013 database was done selecting patients with an admission diagnosis of Achalasia using International Classification of Diseases, Ninth Revision (ICD-9) codes. Esophagomyotomy (E) and Esophagus Dilation (ED) were isolated based on ICD-9 procedure codes. A chi-square analysis was performed to determine variables to be included in a multivariable analysis. A binary logistic regression analysis was used to examine socio-demographic and complication variables, with a significance level of p < 0.001.
Results:
A total of 83,710 patients were identified who had been admitted for achalasia, of which 30,865 (36.9%) received a myotomy and 10,855 (13.0%) received pneumatic dilation. Logistic regression demonstrated that pneumatic dilation was performed more for adults aged 80 or greater, those with Medicaid or Uninsured status, and African American. After incorporating demographic and social variables, those who underwent a pneumatic dilation were more likely to has a hospital course complicated by pneumonia and acute renal failure. Those who underwent a pneumatic dilation were not statistically significantly less likely to have myocardial infarction.
Discussion:
Our data shows that LHM was performed more frequently in younger patients possibly because dilation is associated with higher relapse rates and need for repeat dilations, which may not be preferable in a younger patient. Younger patients are more often surgical candidates and may tolerate LHM better than older. Patients with achalasia who underwent pneumatic dilation more frequently developed pneumonia and acute renal failure but less frequently developed myocardial infarctions compared to those who underwent LHM. We hypothesize that this difference may be due to patients on whom LHM is being performed undergo endotracheal intubation and general anesthesia, which increase the risk of myocardial infarction due to greater cardiac demand. We also noted that pneumatic dilation was preferred in patients that were uninsured or had Medicare, or Medicaid as compared to private insurance payors. Pneumatic dilation is more cost-effective and can be done routinely as an outpatient procedure, helping reduce health care costs for both insurance providers and patients. Resolution of some of these barriers may help shift the paradigm of performing a specific procedure though it is unclear what the cause of these associations are, and further research will benefit providers to help them determine an individualized treatment option for their patients.
Introduction:
Achalasia is an idiopathic esophageal motility disorder that affects roughly 1.6 out of every 100,000 people each year, with rates reported to be increasing each year [1,2]. This disease occurs when there is disappearance of the myenteric neurons at the esophageal wall resulting in spasm or absent lower esophageal sphincter relaxation and peristalsis[3]. As a result, patients tend to present with dysphagia, regurgitation, chest pain, and weight loss[4]. The gold standard for diagnosis of achalasia is high resolution manometry with pressure topography, and with this technique the disease can be classified into three subtypes: type I classic achalasia, type II achalasia with pan-esophageal pressurization, and type III spastic achalasia[5,6]. Treatment options for achalasia include pneumatic dilation, surgical myotomy, peroral endoscopic myotomy, and botulinum injection4. While no treatments reverse the destruction of ganglion cells, they aim to decrease resting pressure at the lower esophageal sphincter[7,8].
Patients typically undergo pneumatic dilation or surgical myomectomy. In pneumatic dilation, the procedure weakens the lower esophageal sphincter by applying force with a balloon, thus stretching the muscle[9]. One of the main risks of pneumatic dilation is perforation. Currently, limited studies favor laparoscopic Heller myotomy over pneumatic dilation[10,11].Surgical intervention is typically recommended for male patients younger than 40 years old, patients with pulmonary symptoms, and those who failed one or two pneumatic dilation[12,13]. One of the main complications of this surgery is severe acid reflux[14].
Currently, there are no established guidelines to decide between undergoing pneumatic dilation versus. laparoscopic Heller myotomy. Our aim with this study was to investigate characteristics of admissions and patient socio-demographics that may influence treatment decisions. Additionally, our study aimed to look at the association between these procedures and post-procedure complications, including pneumonia, urinary tract infection, myocardial infarction, and acute renal failure.
Methods:
Data Source:
The National Inpatient Sample (NIS) database was used to perform a retrospective analysis of the inpatient admissions in the United States. The database is a redacted, publicly accessible database available through the Healthcare Cost and Utilization Project (HCUP), which has been previously researched in this population and justified as an effective measurement of inpatient admissions in the United States [Trieu et al]. This database was queried from January 1, 2001, through December 31, 2013. This database consists of an estimated eight million hospitalizations every year which represent about 20% of the admissions that have occurred in the United States and include about 47 states along with the District of Columbia. This patient population encompasses about 97% of the United States population. The database provides access to key portions of the hospital stay including the primary and associated admission diagnoses, the inpatient mortality, hospital costs, payor source, length of stay, socio-demographics (including median income status, age, sex, race, etc.). Included in this information is also any procedures the patient went through during their stay along with any complications from these procedures. Each admission is given an estimated discharge weight to compute national estimates and is used to correct for sampling error. During the years 2001-2013, the International Classification of Disease, Ninth Revision (ICD-9) codes were used for all diagnoses, procedures, and comorbidities listed in the database. This data is a publicly available, de-identified database, as such due to the nature of this study there was no Institutional Review Board approval required for this study and informed consent was waived.
Study Population:
All of the patients that were aged 18 years or older at the time of their admission, with a primary diagnosis (DX1 – DX3) of achalasia were extracted using ICD-9 diagnosis codes (Supplemental Table 1) from 2001 to 2013. All adults with achalasia who underwent an inpatient Esophagomyotomy (E) and Esophagus dilation (ED) were isolated based on ICD-9 procedure codes (Table 1).

Table 1: ICD-9 Diagnostic and Procedure Codes
Study Variables/Outcomes:
The aim of this study was to understand both pre- and post-operative variables that were associated with the decision to perform either intervention. The All Patients Refined Diagnosis-Related Groups variables that are part of the database and describe each admission were used. The Charlson Comorbidity Index was calculated using the diagnosis codes available as part of the algorithm.
The primary outcome of this study was to assess factors associated with in-hospital morbidity and mortality. Patient demographics including age, race, gender, median income, insurance status, county population size, hospital region, and Charlson Comorbidity Index Score were identified, and propensity matched. Patient complications from intervention including mortality, pneumonia, urinary tract infection, acute renal failure, and myocardial infarction. A composite post operative complications group was also analyzed. Secondary outcomes included hospital length of stay and hospital charges which were provided by the database, extracted, and analyzed.
Statistical Analysis:
Statistical Analysis was performed using SPSS, Version 28.0. NIS weights, predetermined by the HCUP database, were used to generate a population-based estimation from the sample size database. We used Pearson chi-squared tests to compare the categorical comorbidity and socio-demographic variables. We incorporated patient demographic variables in a binomial logistic regression to generate propensity scores. A separate binomial logistic regression was utilized to analyze the complications, specifically the morbidity and mortality associated with achalasia stratifying for patients based on intervention modality adjusted by propensity matching.
Results:
We identified 83,710 patients that were admitted to the hospital for a primary diagnose of achalasia from 2001 to 2013. Of these, 41,990 patients did not undergo any intervention. From the remaining 41,720 patients, 30,865 patients underwent a pneumatic dilation and 10,855 underwent LHM. The average age for patients undergoing dilation was 67 years compared to 50 years for LHM. The average length of stay was only 3 days for those undergoing myotomy compared to 5 days for those undergoing the dilation. On average, those that underwent myotomy had the procedure done within the first 24 to 48 hours of admission compared to those who underwent dilation typically in the 48-72 hours duration. The average cost of hospitalization for a pneumatic dilation was $39,457 compared to $37,472 as a result of a myotomy. There was no mortality from a pneumatic dilation however there was a 0.12% mortality rate with LHM.
Multivariable binomial logistic regression analysis (Table 2) was utilized to compare the socio-demographic variables and create the propensity score for the logistic model analyzing morbidity and mortality. Except for the patients who were aged over 80 years of age who were 90.3% more likely to undergo a pneumatic dilation compared to LHM, the other age groups of 19-29 years, 30-50 years, 51-60 years, and 61-79 years were not statistically significantly different. Compared to Caucasians, those that were self-identified as African American were 45.1% more likely to undergo a pneumatic dilation whereas those of Hispanic, Asian, Pacific Islander, or Native American races were not significant in terms of intervention choice. Self-reported sex did not play a statistically significant role in determining between LHM and dilation. The median income at all quartiles including lowest 25th, 25-50th, 51-75th, and 75th-100th percentile all were not significantly important in determining between the intervention the patient received. Only those counties that had very low populations and were designated as not metropolitan or micropolitan counties were statistically significantly 80% more likely to undergo a myotomy compared to metropolitan areas with greater than one million population. Hospital region was an important factor for determining the type of intervention, the Mountain and Pacific regions were 57% more likely to undergo a myotomy as compared to the Eastern region. Other regions including the Midwest and the South did not have a significant increased odd of one procedure or the other. As compared to Private Insurance, the patients with Medicare were 82% more likely to undergo a myotomy whereas the patients that were uninsured were 61.6% more likely to undergo a pneumatic dilation. Those with Medicaid were not significantly associated with one procedure. When looking at the Charlson Comorbidity Index, as compared to a score of zero, any score greater than zero was associated with increased occurrence of pneumatic dilation. Those with a score of 1 were 41.1% more likely, score of 2 were 82.7% more likely, and a score of 3 or greater 65.2% more likely.
After adjusting for the differences and variables by creating a propensity matched logistic regression analysis, common complications of interventions were analyzed to compare pneumatic dilation to LHM (Table 3). The occurrence of pneumonia was 77.6% more likely to occur in patients undergoing pneumatic dilation as compared to the myotomy. 94.4% of patients undergoing dilation were more likely to have acute renal failure as compared to those with the myotomy. Other complications including urinary tract infections, myocardial infarctions, and a bundle of post operative infections were all not significantly related. It was observed, though not statistically significant, that patients undergoing a dilation were more likely to have urinary tract infections, and post op complications whereas those undergoing myotomy were more likely to have a hospital stay complicated by acute myocardial infarction.
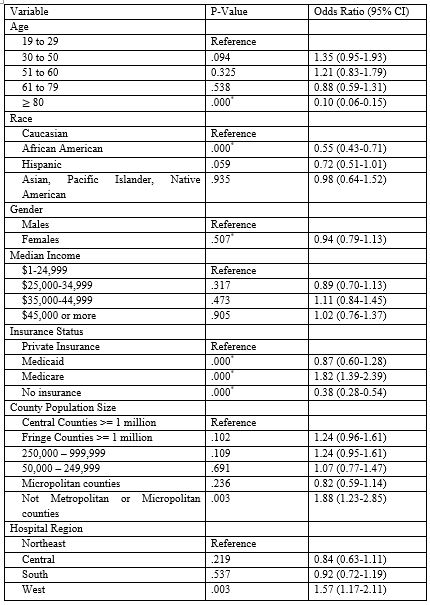
Table 2: Predictors of Myotomy (1) over Pneumatic Dilation (0)
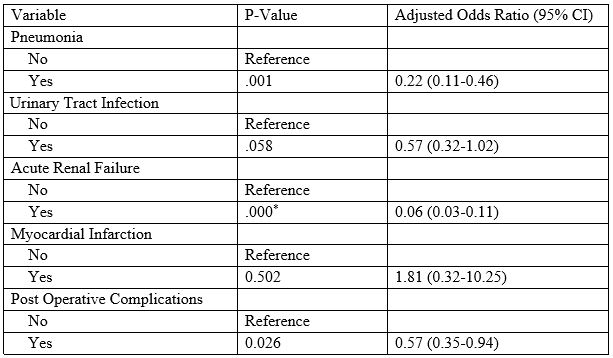
Table 3: Predictors of Myotomy (1) over Pneumatic Dilation (0)
Discussion:
In this study we examined the factors that influenced the decision to treat achalasia with LHM versus pneumatic dilation as well as common complications from both procedures. We found that patients over the age of 80 years, with Medicare or no insurance and those admitted over the weekend were all more likely to undergo pneumatic dilation. LHM was more frequently performed than pneumatic dilation and was associated with a lower risk of pneumonia, UTI, or acute renal failure. However, it was associated with an increased risk of myocardial infarction and was the only intervention that had associated mortality. It was observed that compared to having a Charlson Comorbidity Score of 0, any complication or higher age was associated with performing a pneumatic dilation more frequently than a myotomy.
Pneumatic dilation has been shown to be more cost effective than LHM, particularly over the short term[15–17]. It also takes less time compared to LHM. In addition to these benefits, the risk of perforation is also low with pneumatic dilation[18,19]. Combined, these factors may make pneumatic dilation a favorable option for patients who do not have private insurance. In elderly patients, achalasia has been shown to present with decreased lower esophageal sphincter (LES) pressures and may be a result of a loss of peristalsis [20–22]. Given that elderly patients carry higher surgical risk, have a different underlying cause of achalasia, and may not require a long-term solution, they are more likely to benefit from pneumatic dilation over LHM. Finally, the weekend effect has been well documented, especially with regard to endoscopic procedures such as upper gastrointestinal endoscopy for an upper gastrointestinal bleed[23–25]. Given increased time to respond as well as fewer resources available on the weekend or off hours, pneumatic dilation can serve as either a temporary solution for a patient awaiting further evaluation or a long-term solution for a patient who is a high-risk surgical candidate, making it a suitable treatment approach over the weekend and during off-hours.
Despite the fact that LHM is a more invasive procedure, we found that the risk of pneumonia, UTI and acute renal failure was lower compared to that of pneumatic dilation. However, it has been shown that LHM typically provides a long-term solution for achalasia and provides more benefit for younger, healthier patients who can tolerate surgery[26–28]. While age alone has not been shown to be an independent risk factor, older patients typically have more comorbidities that increase their surgical risk [29–31]. The increased risk at baseline of patients undergoing pneumatic dilation may present a bias toward higher rates of complications in these patients. Unlike pneumonia, UTI and acute renal failure, myocardial infarction is likely a direct result of anesthesia. EGDs typically requires less sedation and less time under anesthesia and has been found to be relatively safe even in the setting of an active myocardial infarction[32–34]. Additionally, patients undergoing pneumatic dilation are typically not surgical candidates, and understanding risks associated with procedures allows some preparation to be made before intervening.
There are important limitations to consider in this study. First, the NIS database only contains data for an individual hospitalization and may not capture patients who were discharged quickly and returned to the hospital. It also relies on accurate coding of diagnoses and procedures performed, and errors in coding may affect the results. Finally, the NIS does not allow for a temporal relationship to be established, making it difficult to assess cause and effect. Further studies analyzing post pneumatic dilation outcomes may clarify the relationship between complications from this study and pneumatic dilation versus LHM.
Conclusion:
In conclusion, we found that LHM had a lower risk of associated pneumonia, UTI, and acute renal failure compared to pneumatic dilation. We also showed that primary payor, age, and comorbidity index played a significant role in determining the type of procedure a patient received. As new methods for treating achalasia are developed, further research into risks will be needed to determine the best option for individual patients based on their unique risks. Additionally, further research into other determinants of health, such as the weekend effect and primary payor may demonstrate biases that need to be addressed in the care of patients with achalasia.
Conflict of interest
All authors have no conflict of interests to declare.
Acknowledgements
We have no acknowledgements.